Mineralogy and Petrology – Engr. Emily
1/216
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
217 Terms
Mineralogy
Science of minerals: their properties, classification, occurrence, and identification.
Crystallography
Study of crystal structures, atomic arrangements, and how these relate to material properties.
Polymorphs
Minerals with the same chemical composition but different crystal structures (e.g., diamond vs graphite).
Form (crystal form)
Group of crystal faces with the same relation to the crystal’s symmetry elements.
Habit (crystal habit)
Outward appearance of a crystal or aggregate; influenced by growth conditions.
Amorphous solid
Solid lacking ordered crystal structure (e.g., glass, obsidian).
Crystalline solid
Solid with a well-ordered, repeating atomic arrangement.
Anisotropic
Exhibiting direction-dependent properties (e.g., hardness, refractive index).
Isotropic
Properties are the same in all directions.
Crystal
Solid having a regularly repeating Arrangement of atoms. Crystalline Structure.
Crystal lattice
3D array of points representing the periodic arrangement of atoms in a crystal.
Unit cell
Smallest repeating unit that fully describes the crystal structure. Fundamental elementary pattern, “building blocks”.
Primitive lattice (P)
Lattice with atoms only at the corners of the unit cell.
Body-centered
Lattice with atoms at the corners and in the body center.
Face-centered
Lattice with atoms at the corners and at the centers of each face.
Base-centered
Lattice with atoms at the corners and at the centers of the base faces (top and bottom)
Interfacial/Interaxial angles
Angles between adjacent crystal faces.
Lattice constants
Edge lengths along the principal axes of a crystal lattice.
Crystallographic Axes
Set of reference axes in a crystal that are used to describe the crystal systems.
Euler’s Formula
# of faces + # of solid angle = # of edges + 2
Crystal System
Refers to the geometry of crystal structuree. It is described by crystallographic axes.
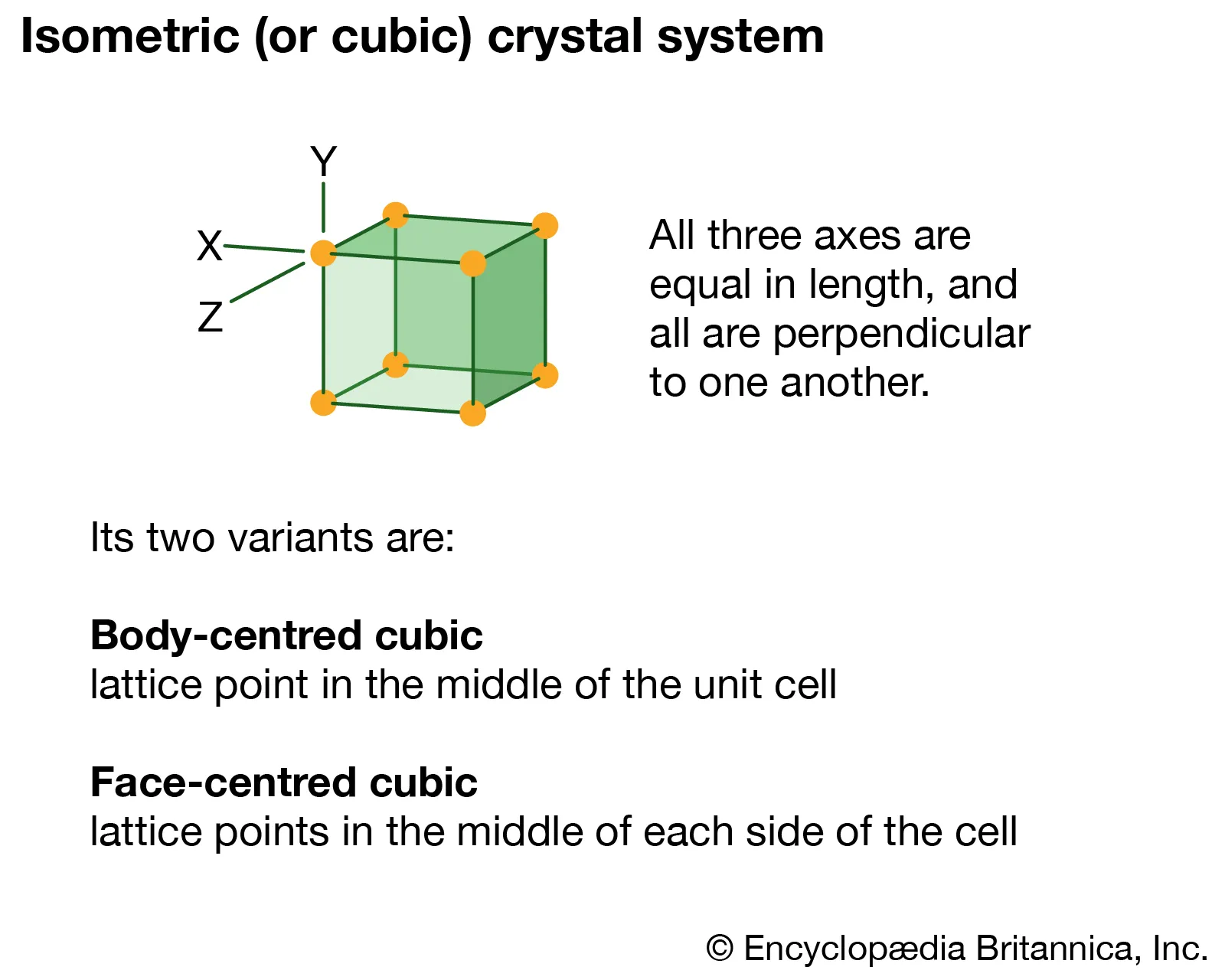
Isometric/Cubic
System where three axes are equal; high symmetry (example: halite).
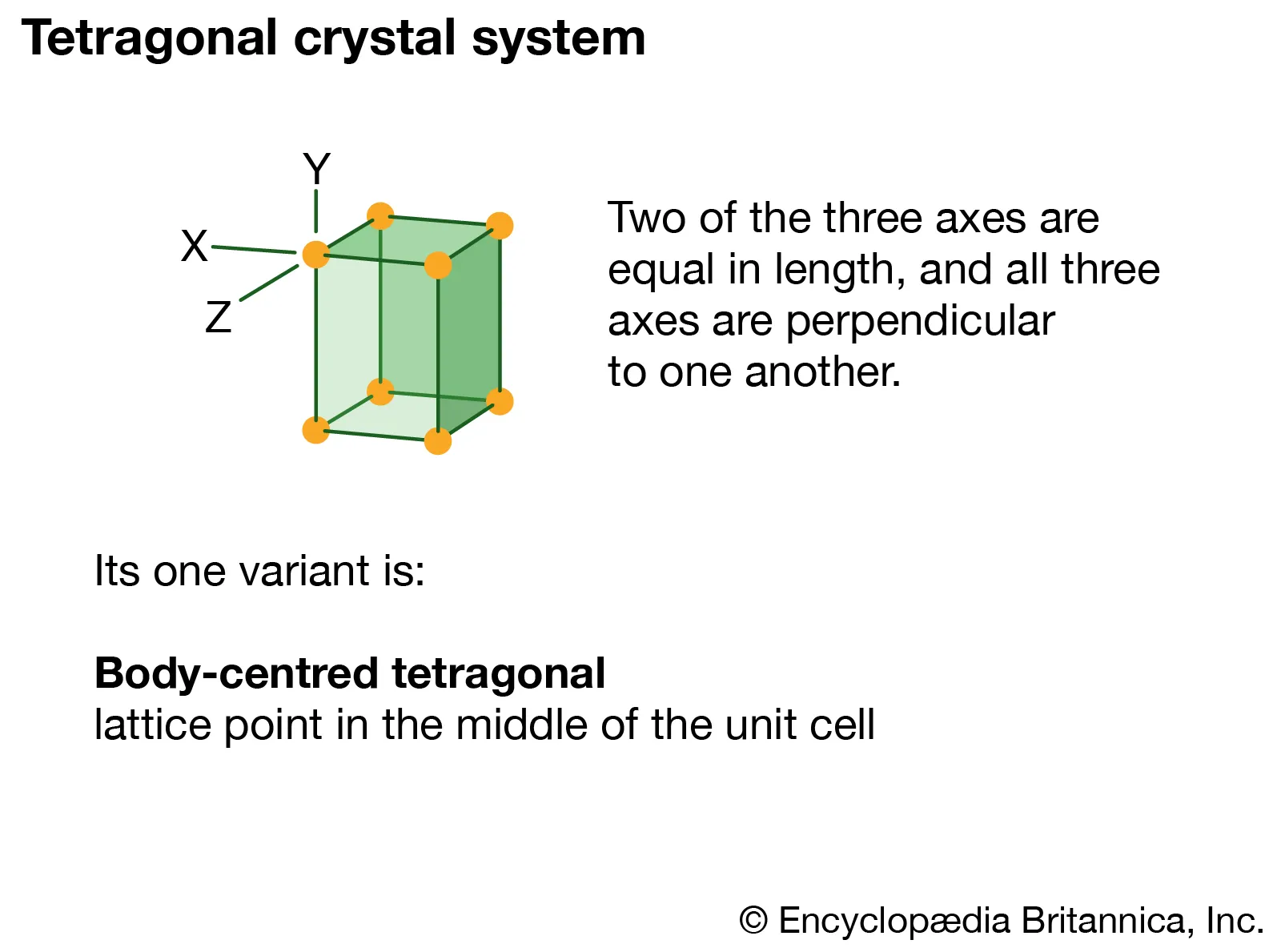
Tetragonal
Three axes, all perpendicular; two axes equal in length, one distinct. (Ex. Apophyllite)
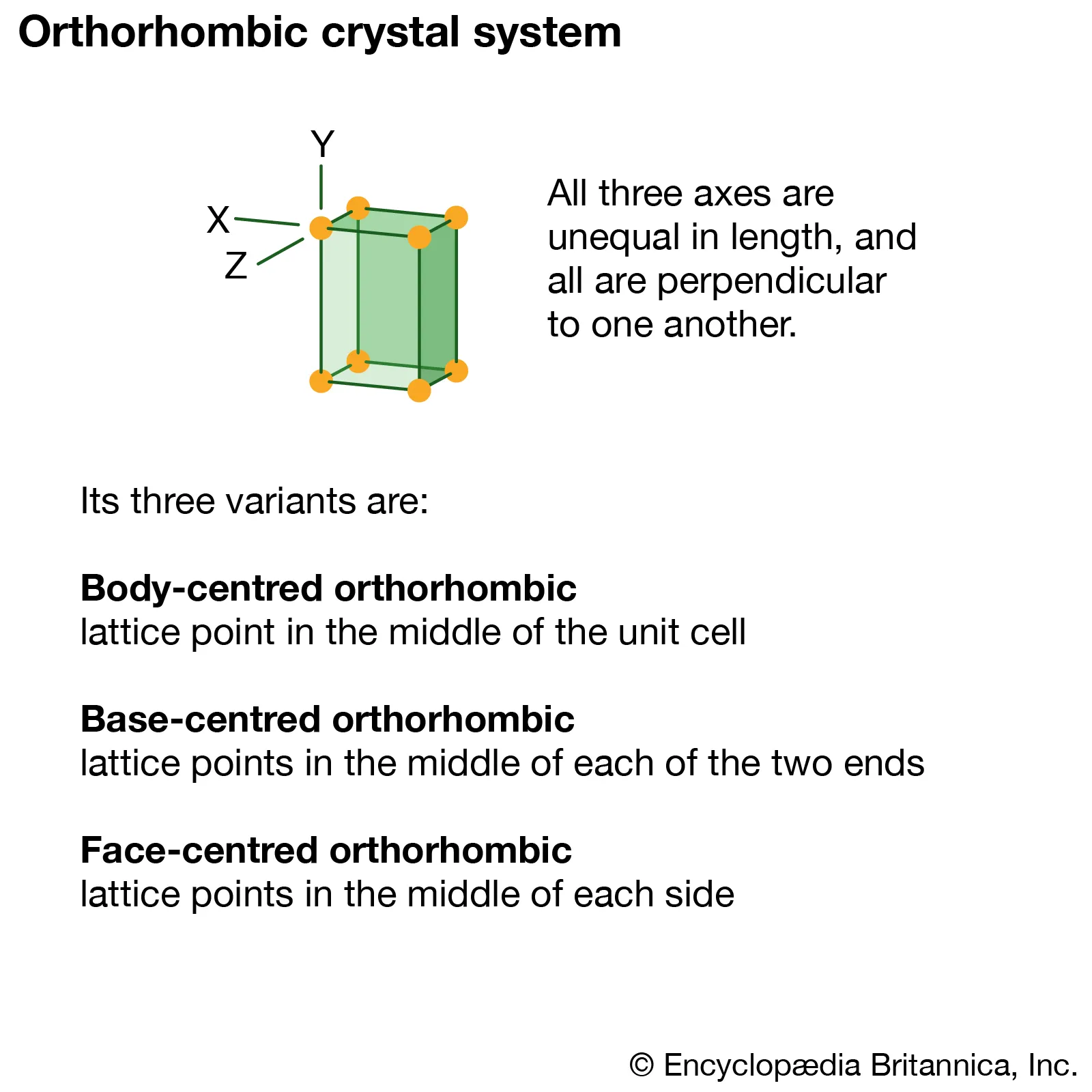
Orthorhombic
Three mutually perpendicular axes of different lengths.
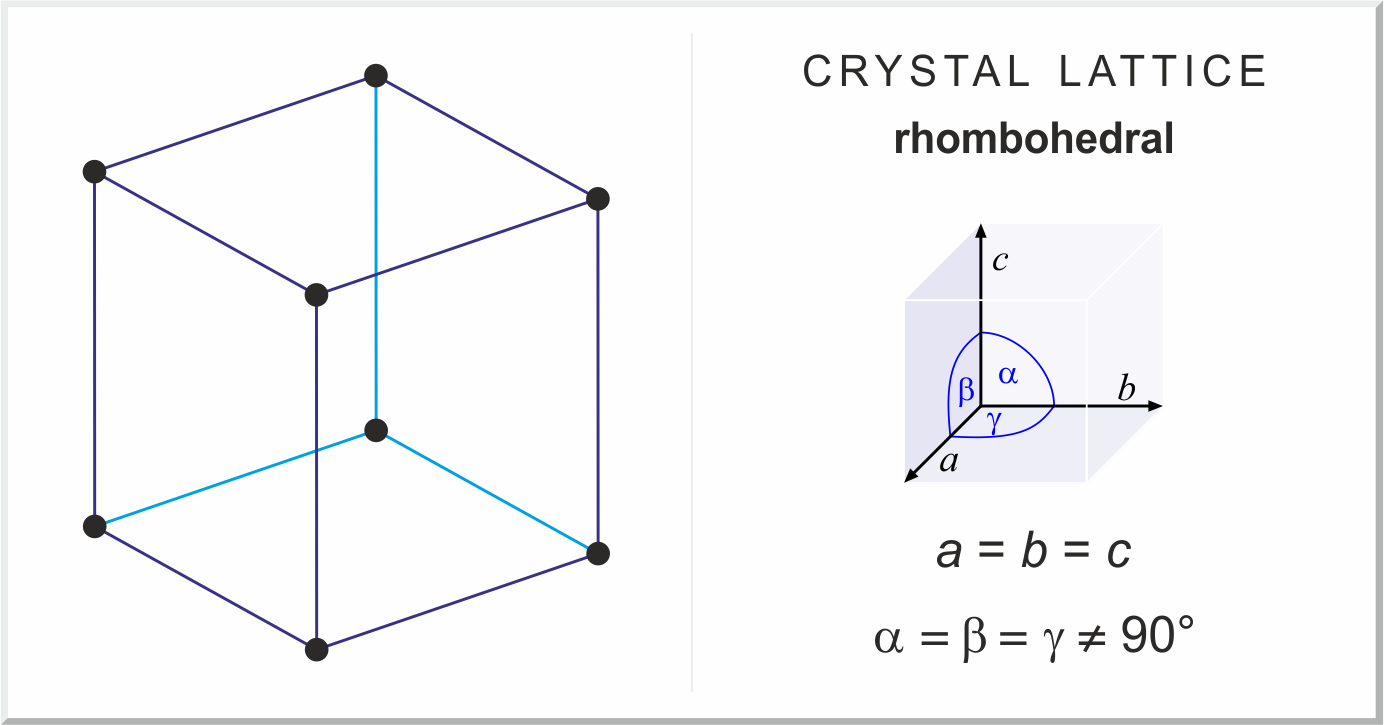
Trigonal/Rhombohedral
Three axes of equal length. No perpendicular lengths (Ex. Calcite)
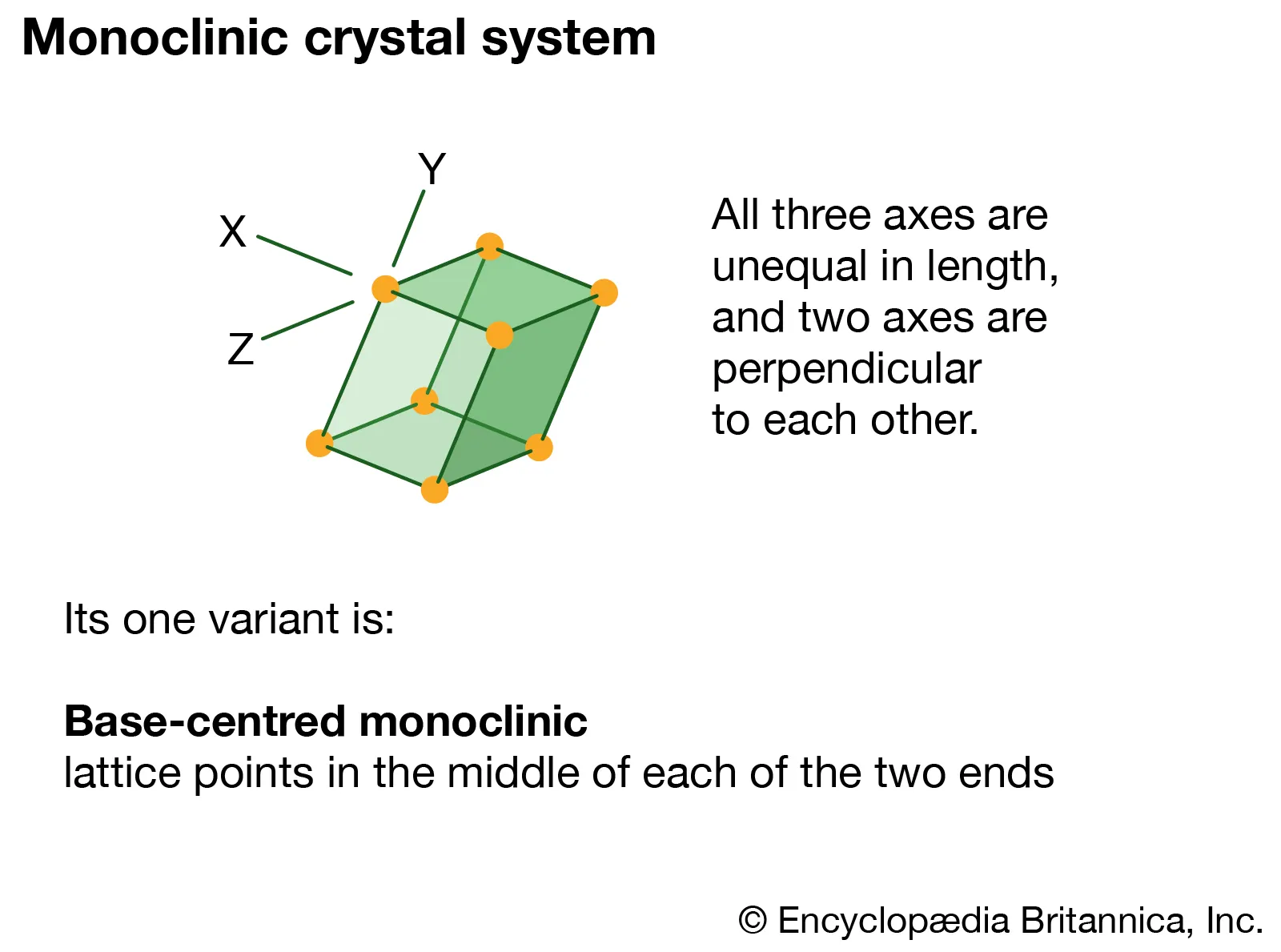
Monoclinic
Three axes, two not perpendicular; one angle non-90°.
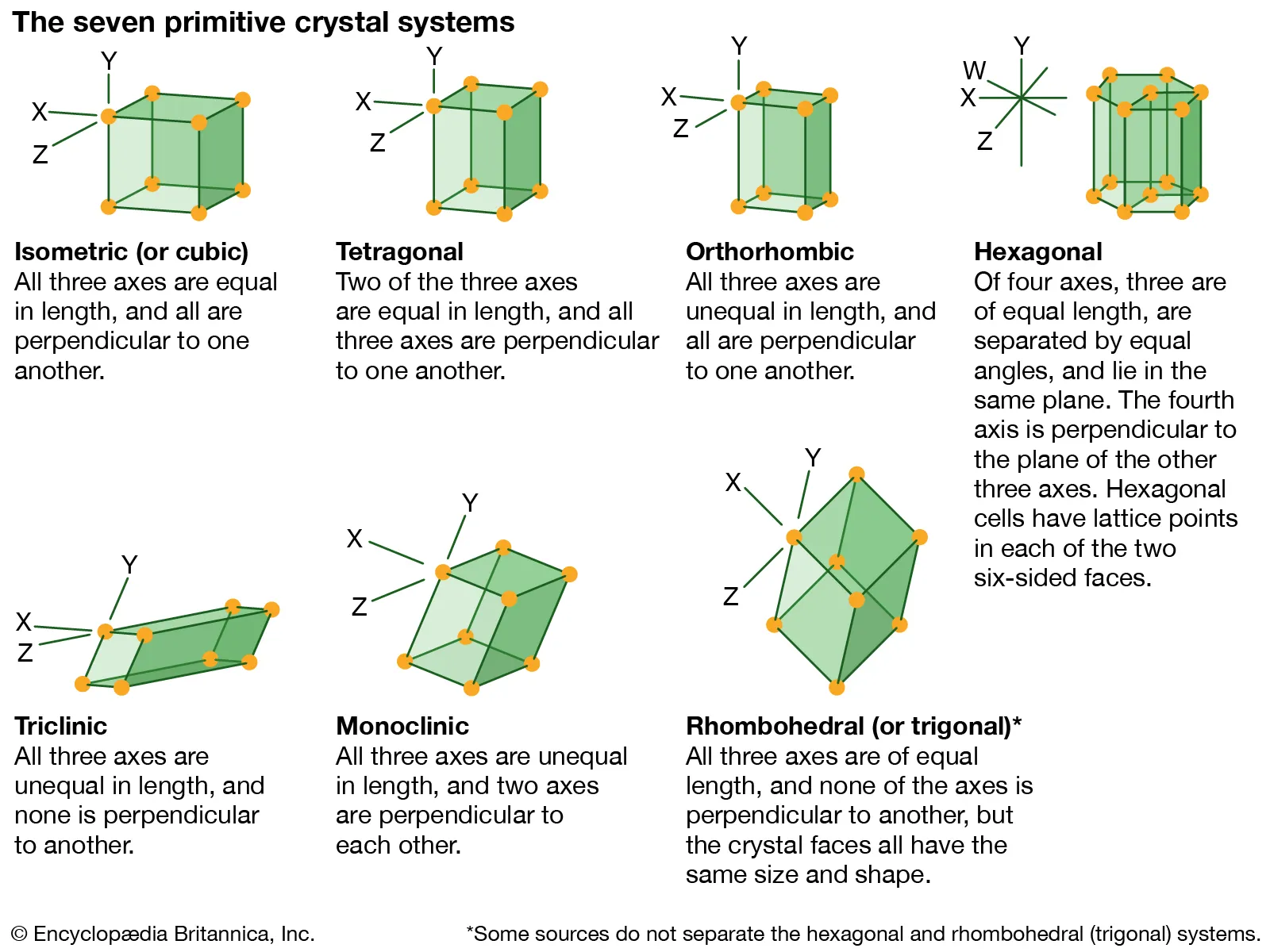
Hexagonal
Four axes with three in one plane 120° apart; one perpendicular axis to the other 3 lengths.
Bravais lattices
Distinct lattice types: Primitive (P), Body-centered (I), Face-centered (F), and others (C, etc.).
Crystal forms
Closed vs open forms; the set of crystal faces that define a form.
Symmetry element
Geometric feature (plane, line, or axis) used to describe symmetry in crystals.
Translational Symmetry
Symmetry accompanying the translation or movement of a plane or unit cell without rotation.
Glide Planes
Symmetry that involves translation parallel to the glide planes, followed by reflection across the glide planes.
Screw Axis
Translation parallel to the screw axis, followed by rotation about the screw axis.
32 Point Groups
Classification of crystal symmetry excluding translational symmetry.
Space group
Combination of crystal symmetry operations including translations; 230 known space groups.
Axis of Symmetry
Line about which the crystal may be rotated so as to show the same view of the crystal more than once per rotation
Diad
2 fold axis; when rotating the same face/view occurs 2 times
Triad
3 fold axis; when rotating the same face/view occurs 3 times
Tetrad
4 fold axis; when rotating the same face/view occurs 4 times
Hexad
6 fold axis; when rotating the same face/view occurs 6 times
Schoenflies Notation
Usually used in spectroscopy. Enough to describe symmetry of molecule.
Hermann-Maguin Notation (International Notation)
Can describe the symmetry of elements in point groups, plane groups, and space groups of crystal lattice.
Crystal growth
Expansion of a crystal as atoms/ions attach to its surfaces.
Nucleation
Formation of a stable seed (nucleus) from which crystals grow.
Heterogeneous nucleation
Nucleation aided by surfaces, impurities, or artificial seeds.
Slow cooling method
Crystallization by cooling a saturated solution slowly; solubility decreases with temperature.
Slow evaporation method
Crystallization by gradual solvent removal to precipitate solute.
Temperature gradient method
Crystallization driven by transport from hot to cold regions, causing deposition.
Critical nucleus radius
Minimum stable size a nucleus must reach to continue growth.
Crystallization
Process through which atoms, molecules, or ios arrange themselves in a repeating pattern
Supercooling
the cooling of a liquid below its equilibrium freezing or crystallization temperature without the immediate formation of solid crystals.
Euhedral
Crystal faces are well-formed and clearly developed.
Subhedral
Crystal faces are partially developed; some faces are well-formed.
Anhedral
Crystal with no well-formed crystal faces.
Crystal habits
Common shapes crystals can take, e.g., acicular, tabular, platy, prismatic.
Prismatic
Long and slender, with well-formed prism faces (Beryl, Tourmaline)
Tabular
Crystals are flat and plate-like, resembling a tablet (Barite, Apophyllite)
Equant
Crystals have roughly equal dimensions in all directions, often forming blocky shapes (Garnet, Halite)
Acicular
Crystals are very thin, slender, and needle-like (Natrotile, Rutile)
Fibrous
Crystals appear as fine threads or fibers (Chrysotile, Asbestos)
Capillary
Crystals are extremely thin and hair-like, often finer than fibrous types. (Millerite)
Bladed
Crystals are flat and elongated, shaped like knife blades (Kyanite)
Columnar
Crystals are thick and vertical, resembling pillars or columns; tourmaline, pyroxene, and augite
Lenticular
Crystals are lens-shaped and rounded, like in siderite and hematite
Filiform
Crystals grow as flexible wire-like threads, typical of native copper and native silver

Dendritic habit
Arborescent, tree-like crystal growth with branched forms, like pyrolusite, manganese oxides, and silver.

Reticulated habit
Lattice-like, networked crystal aggregation

Drusy habit
Surface covered with a dense layer of minute crystals.
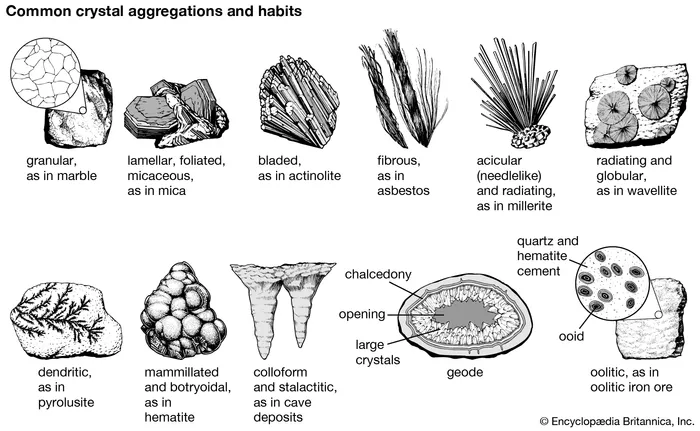
Mammillary habit
Large, rounded masses formed by radiating growth.
Radiating
Crystals grow outward from a central point, found in pyrite, natrolite, and wavellite
Roseiform
Crystals cluster in a flower-like shape, as seen in barite (desert rose) and gypsum rose
Botryoidal
Crystals form rounded, grape-like masses, common in malachite, hematite, and smithsonite.
Reniform
Crystals form kidney-shaped, curved surfaces, typical of hematite and goethite.
Globular
Crystals form spherical clusters, seen in prehnite and smithsonite.These clusters often resemble small balls or globes.
Oolitic
Rock made of tiny, spherical grains like fish eggs, common in limestone and iron-rich sediments.
Pisolitic
Like oolitic but with larger rounded grains, found in bauxite and limonite
Stalactitic
Hanging, icicle-like mineral growths, formed by calcite, aragonite, and malachite in caves
Geode
Hollow rock cavities lined with inward-growing crystals like quartz, amethyst, and calcite
Massive
A dense, compact form with no visible crystals, typical of magnetite, chalcopyrite, and galena
Granular
Made up of small, equant interlocking crystals, seen in dolomite, olivine, and calcite.
Plumose
Crystals show a feather-like or plume-like pattern, often radiating in delicate, curved arrangements
Lamellar
Crystals form in stacked, sheet-like layers, as in graphite, hematite, or chlorite.
Foliated
Thin, flexible layers of crystals that peel easily, seen in micas like biotite and muscovite
Bonding (ionic)
Electrostatic attraction between oppositely charged ions; high melting points.
Bonding (covalent)
Electron sharing between atoms; common in nonmetals.
Bonding (metallic)
Delocalized electrons bonding metal ions; gives metallic conductivity.
Native elements
Minerals consisting of a single element in pure form (e.g., Cu, Au, Ag).
Sulfides
Minerals composed of sulfur bonded to a metal cation (e.g., pyrite, galena).
Sulfates
Minerals with sulfate (SO4)2- groups bonded to metals.
Oxides
Metal cations bonded with oxygen; hard, dense minerals (e.g., hematite, magnetite).
Hydroxides
Minerals with hydroxide groups (OH-); often hydrated oxide forms.
Halides
Minerals containing halogen ions (Cl-, F-, Br-), such as halite (NaCl).
Silicates
Most abundant group; SiO4 tetrahedra form various structures (nesosilicates to tectosilicates).
Carbonates
Minerals containing CO3 groups (e.g., calcite, dolomite).
Phosphates
Minerals with phosphate groups (e.g., apatite).
Nesosilicates (Olivine group)
Isolated SiO4 tetrahedra; olivine with Forsterite and Fayalite as endmembers.
Inosilicates (Pyroxene group)
Single/double chain silicates; includes pyroxenes like enstatite and augite.
Amphiboles (Double chain)
Inosilicate group (e.g., hornblende) with two chains; commonly hydrous.
Phyllosilicates (Micas)
Sheet silicates; forms mica group (Biotite, Muscovite).
Tectosilicates (Framework)
3D framework silicates; includes feldspars and quartz.