APChem Ch 11 - Intermolecular Forces
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
33 Terms
intermolecular forces
forces of attraction between molecules
intermolecular forces vs INTRAmolecular corces
INTER: between molecules; weaker; define physical properties and states of matter
INTRA: between bonds of atoms; very strong; define chemical properties
Gas State of Matter
indefinite volume and indefinite shape. Molecules are rapidly moving and spread far apart. Highly compressible- expands to fill container; Kinetic Energy keeps them apart
**intermolecular forces are broken
Liquid State of Matter
definite volume, indefinite shape > takes shape of container
**intermolecular forces are still together but are constantly changing and reforming
Solid State of Matter
Definite shape and volume
**intermolecular forces are strong and hold particles in fixed positions
types of IMFs and order of strength
(weakest) London Dispersion < induced-dipole < dipole-dipole < hydrogen bonding < ion-dipole
**first 3 are van der Waals forces and LDFs +induced-dipole are generally same thing
Dispersion Forces (LDFs and induced dipole)
- in all molecules but most significant in nonpolar molecules
- nonpolar particle is temporarily polarized (induced dipole) due to constantly moving electrons within atomic radius
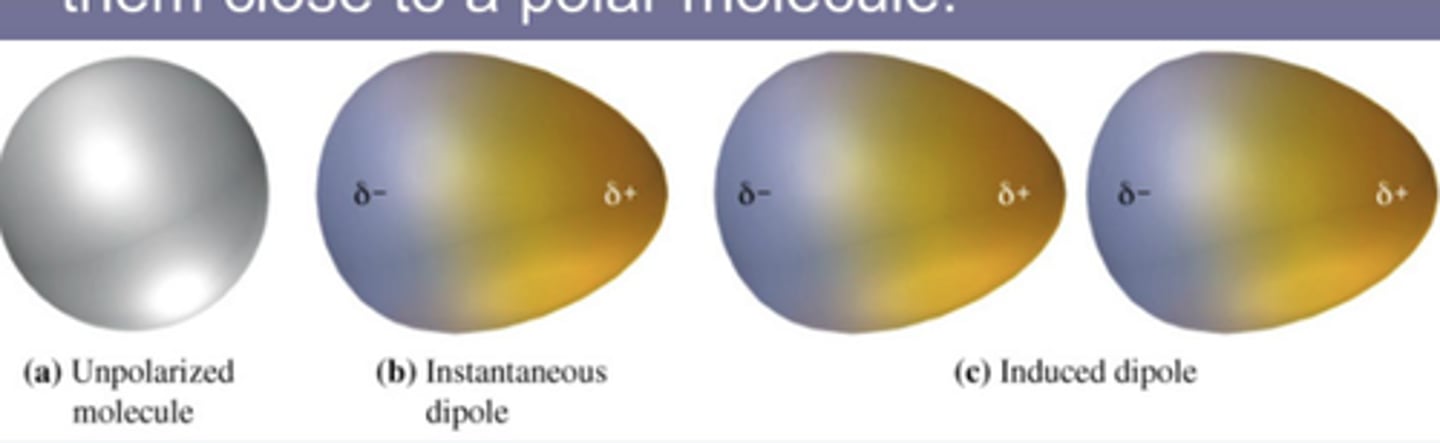
Polarizability
the tendency of an electron cloud to distort
**based on molar mass and # of electrons; ie, bigger molecule = more polarizable = stronger IMFs
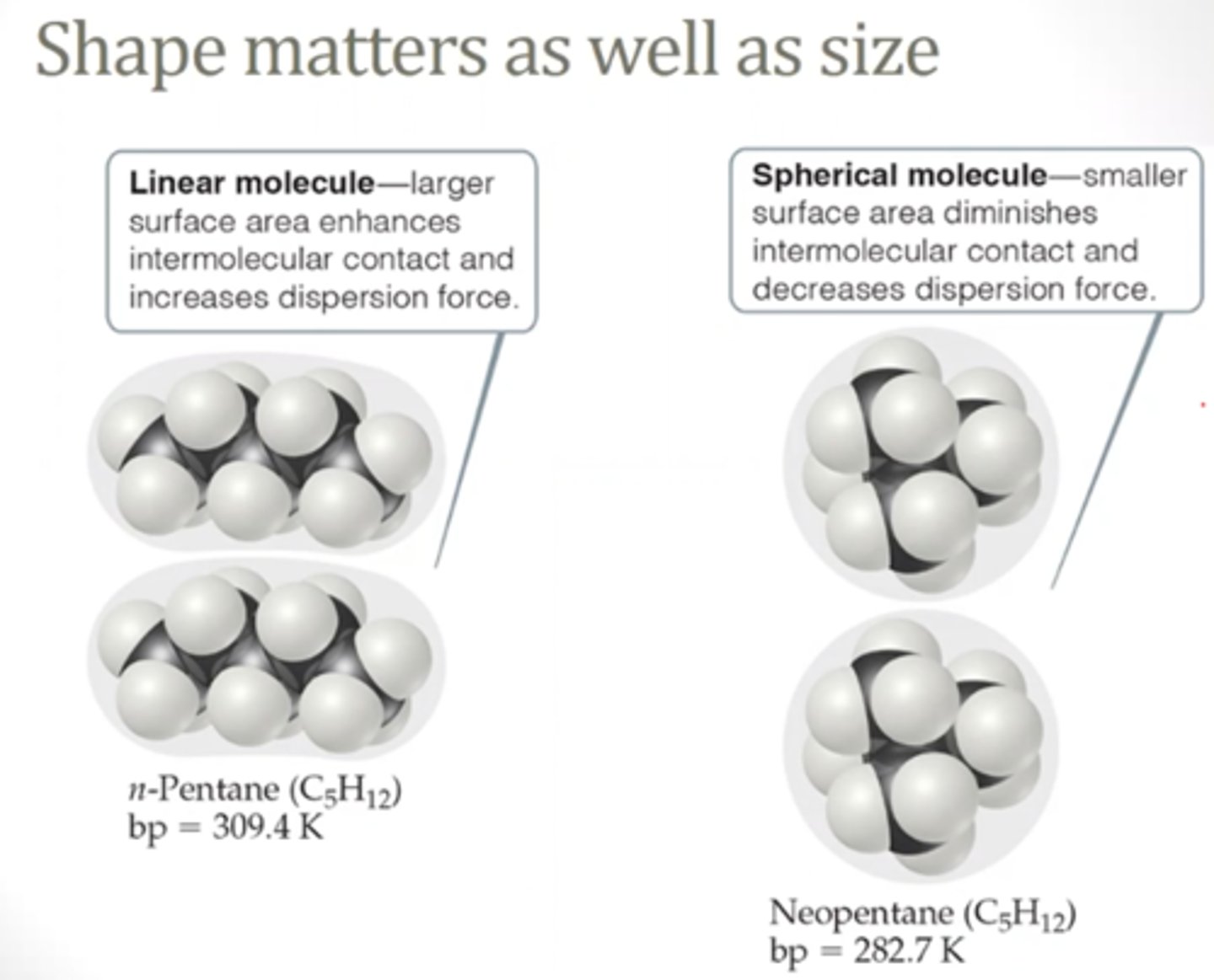
Strength of Dispersion Forces
- based on molar mass/size of molecule
- if similar molar mass (ex: isomers) > depends on shape
- more surface area = stronger dispersion force
dipole-dipole forces
- between two polar covalent molecules
- opposites attract
- more polar (ie higher electronegativity difference) = stronger dipole-dipole force
greater effect between dip-dip and dispersion forces?
if dipole-moment is larger but has smaller BOILING point than a smaller molecule, that means dispersion forces are dominating force
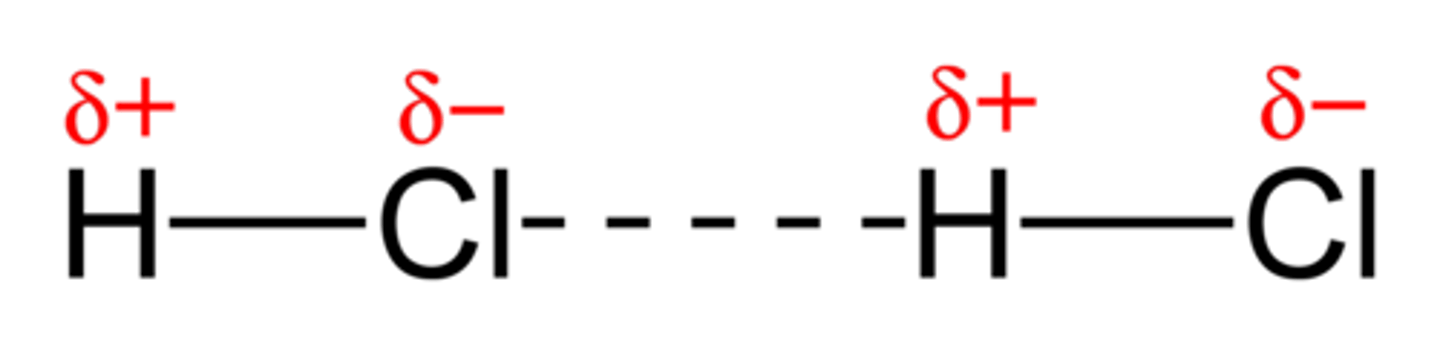
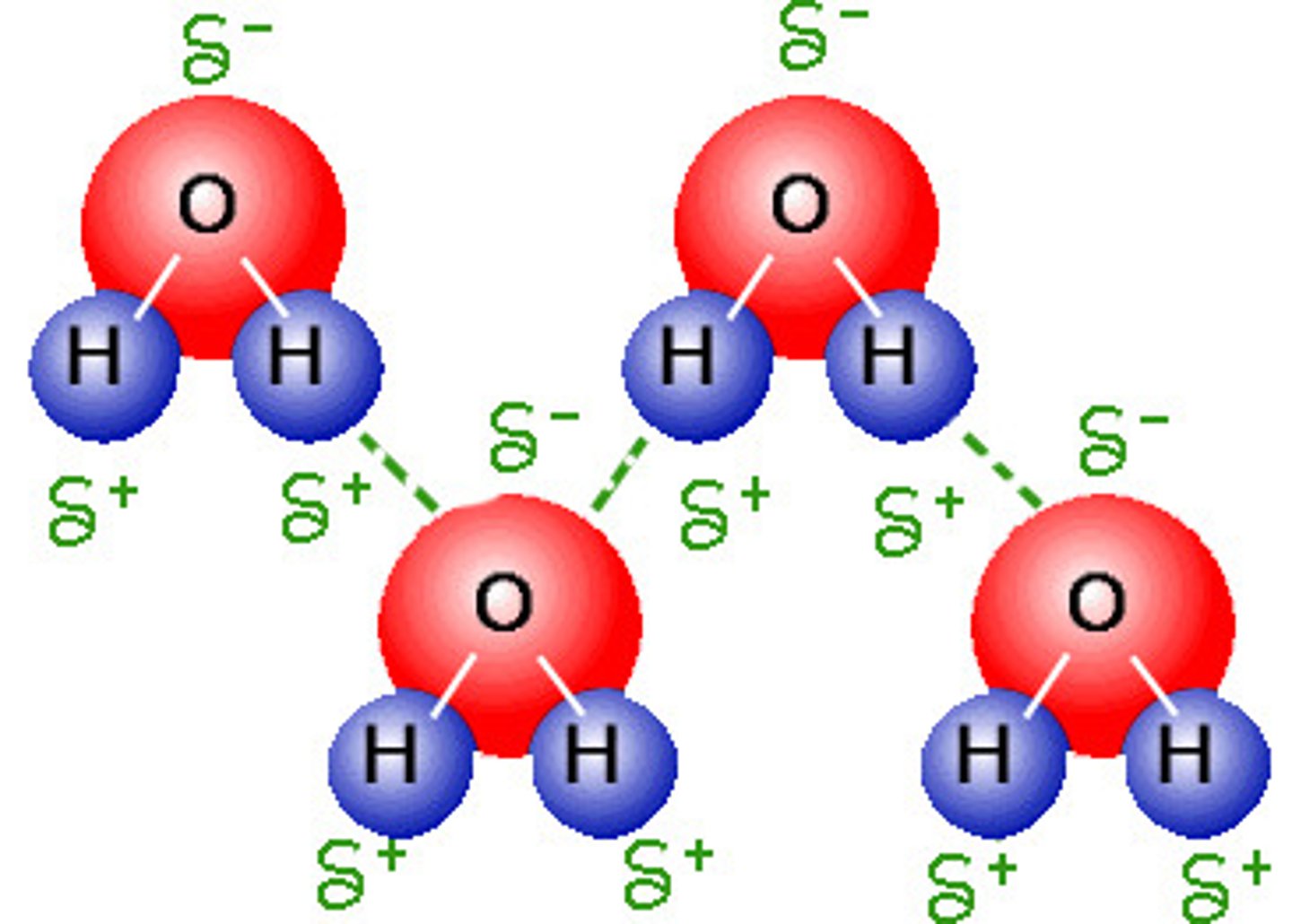
hydrogen bonding
- strong IMFs of H bonded to F,O, N
- H has a "bare" proton (no inner electrons) strongly attracted to highly elecotrnegative F, O, N and therefore can bond very closely
**make sure to draw lewis structure to verify hydrogen bonding
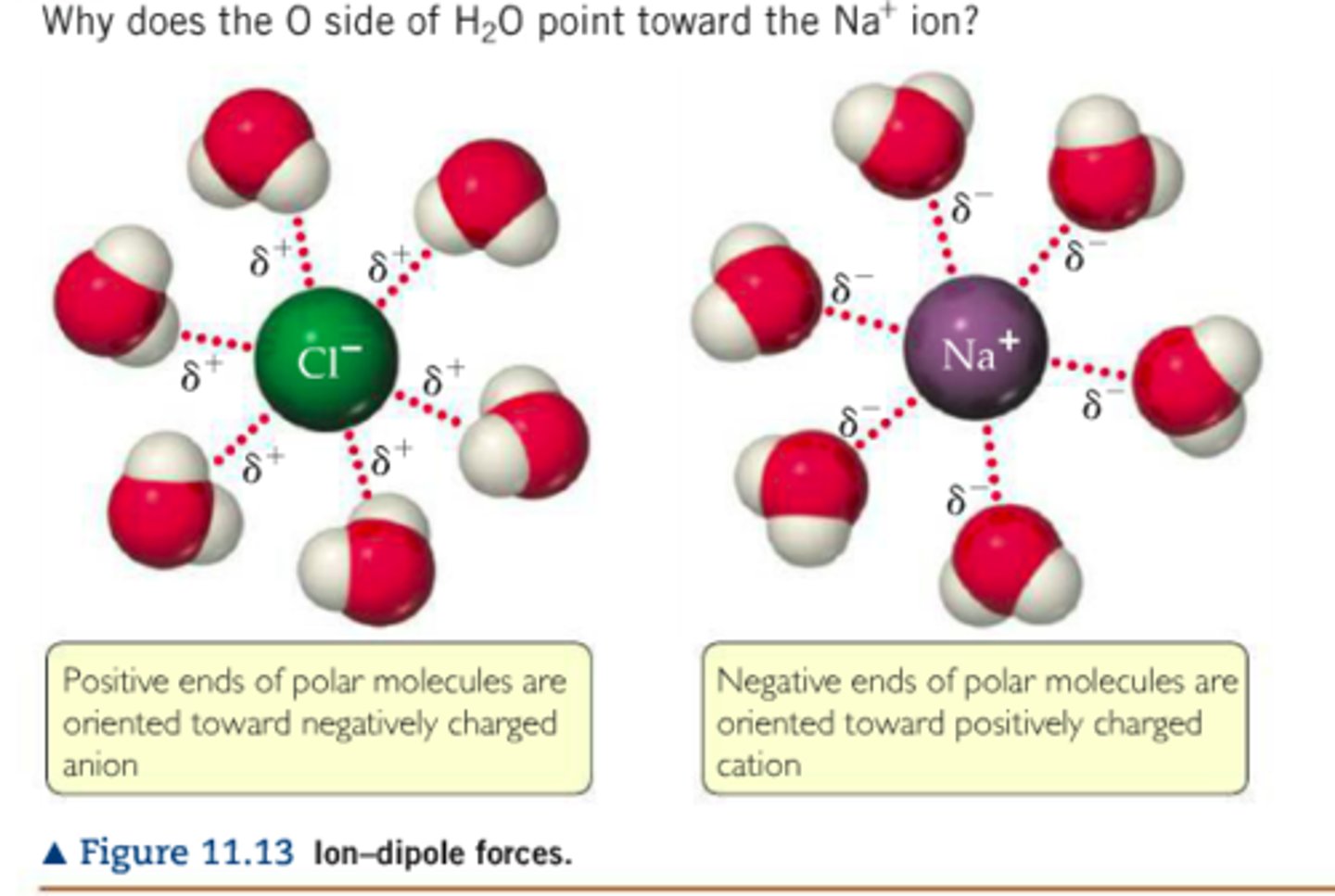
ion-dipole forces
- found in primarly solutions
- between ions and a polar covalent molecule
- ex: NaCl and H2O
- note: NaCl by itself has no IMFs, only ionic bonding
Direct Relationships between IMFs and Properties
when strength of IMFs increase these also increase;
bpt, mpt, surface tension, capillary action, viscosity
Inverse Relationships between IMFs and Properties
when strength of IMFs increase these decrease:
volatility, vapor pressure
bpt/mpt and IMFs
IMFs stronger = molecules held together more strongly = more kinetic energy required to move molecules away from each other for phase changes
viscosity and IMFs
- A liquid's resistance to flowing
- stronger IMFs = more attraction between molecules = more friction between molecules = higher resistance to flow
- others: more polar, bigger molecule/molar mass = higher viscosity
- lower viscosity when higher temperatures because Kinetic energy reduces attraction between molecules

surface tension and IMFs
- energy required to increase the surface area of liquid by a unit amount
- inward attraction of surface molecules
- stronger IMFs = stronger attraction = greater amount of energy to increase surface area
- high temperature reduces surface temperature because high kinetic energy overcomes IMFs
cohesive and adhesive forces
cohesive=forces of a substance to stick to itself
adhesive=forces of a substance to stick to other surfaces
**solids have coh. forces no adhesive forces
liquids have both
gases have neither
capillary action
- ability of liquid to flow in narrow spaces
- adhesive forces are stronger than cohesive forces
- stronger IMFs = greater adhesive forces = greater capillary action
volatility and IMFs
- tendency to vaporize
- stronger IMFs = stronger attraction = more energy required to break bonds between molecules = lesser volatility
vapor pressure
- pressure exerted by vapor when in equillibrium with liquid (or solid) phase
- VAPORIZATION must be in equilibrium with CONDENSATION
- **closed container
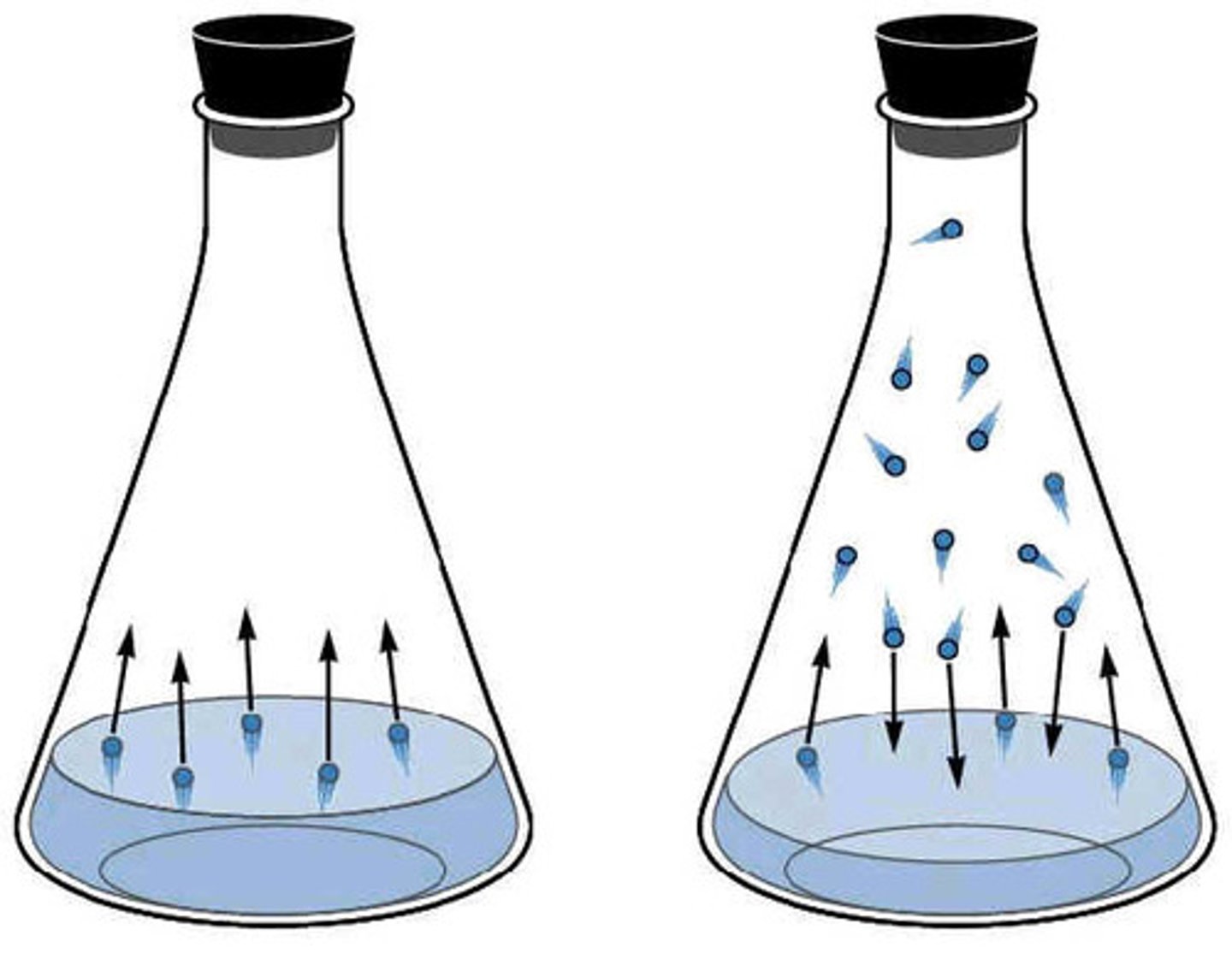
vapor pressure and IMFs
- stronger IMFs = stronger attraction btwn molecules = more energy to vaporize = less vapor exerted = lesser vapor pressure
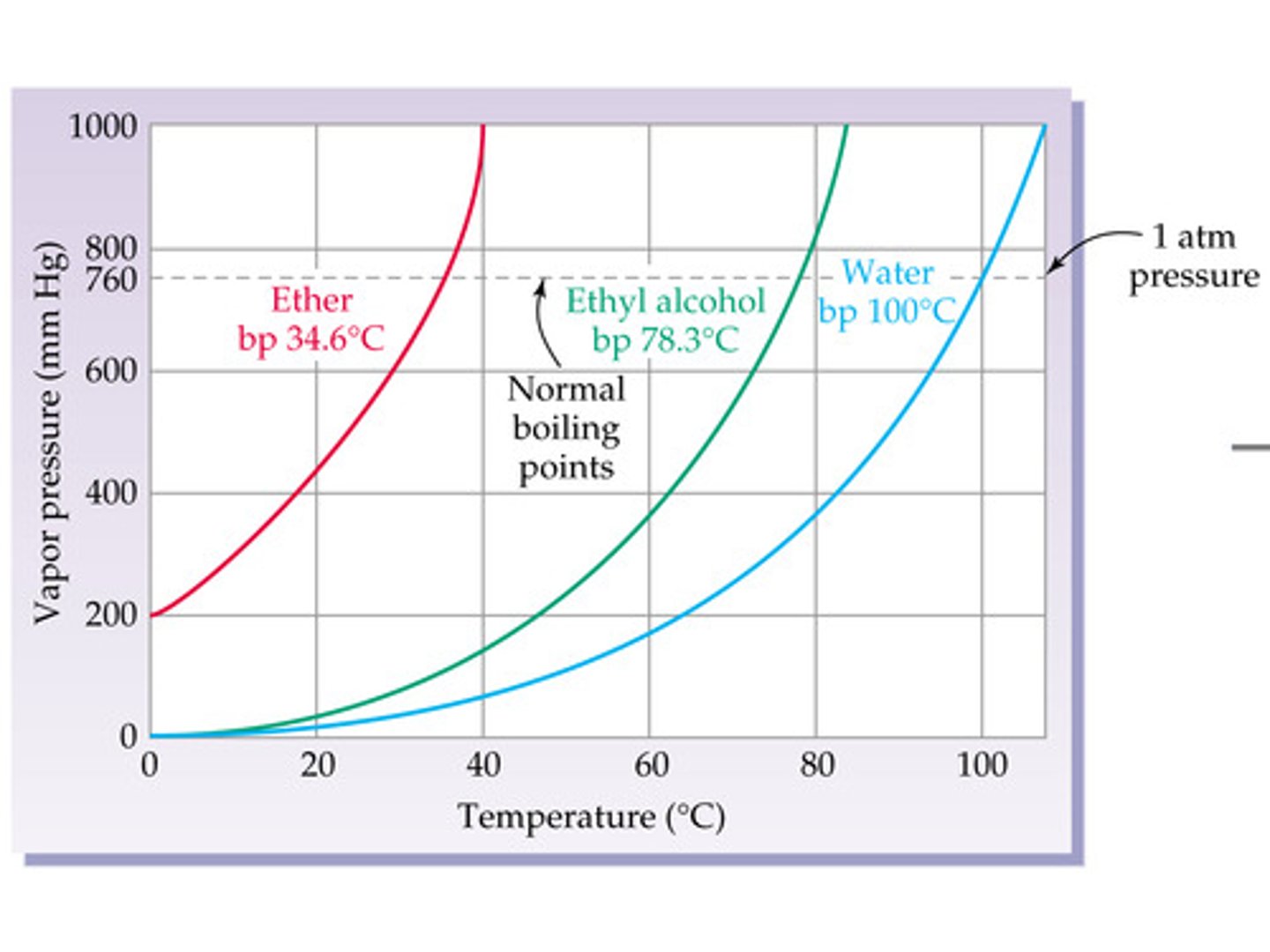
normal boiling point
at standard pressure
**1 atm = 760 torr = 760 mmHg = 101.325 kpa
Phase Changes
+ sublimation is solid STRAIGHT to gas
> opposite is deposition
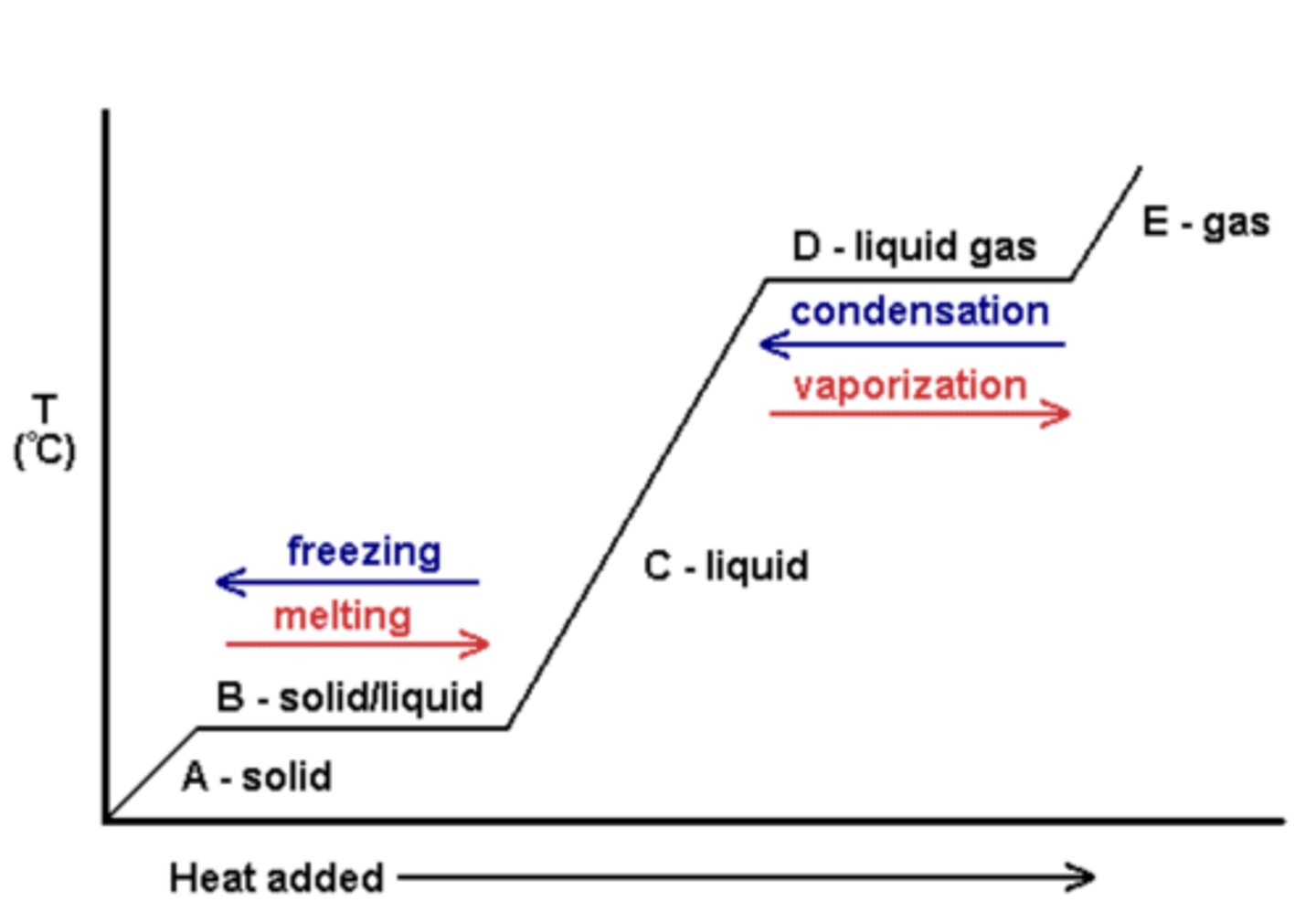
Heat of Vaporization > Heat of Fusion
- more energy is required to change liquid into gas because IMFs must be completely broken
Why is temperature constant during phase changes?
energy is used to break or form bonds rather than change the temperature
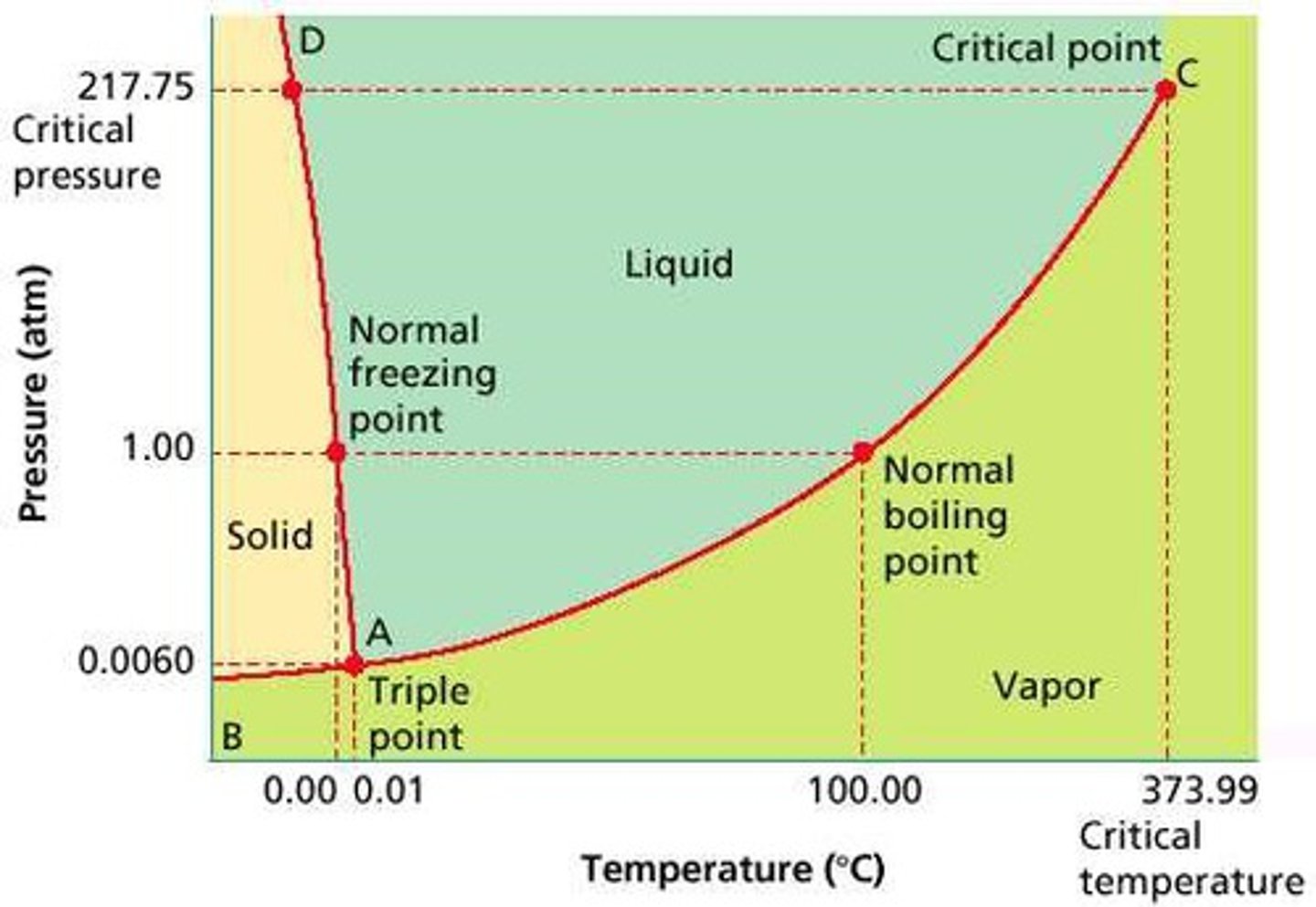
Supercritical Fluids
gas and liquid states become indistinguishable
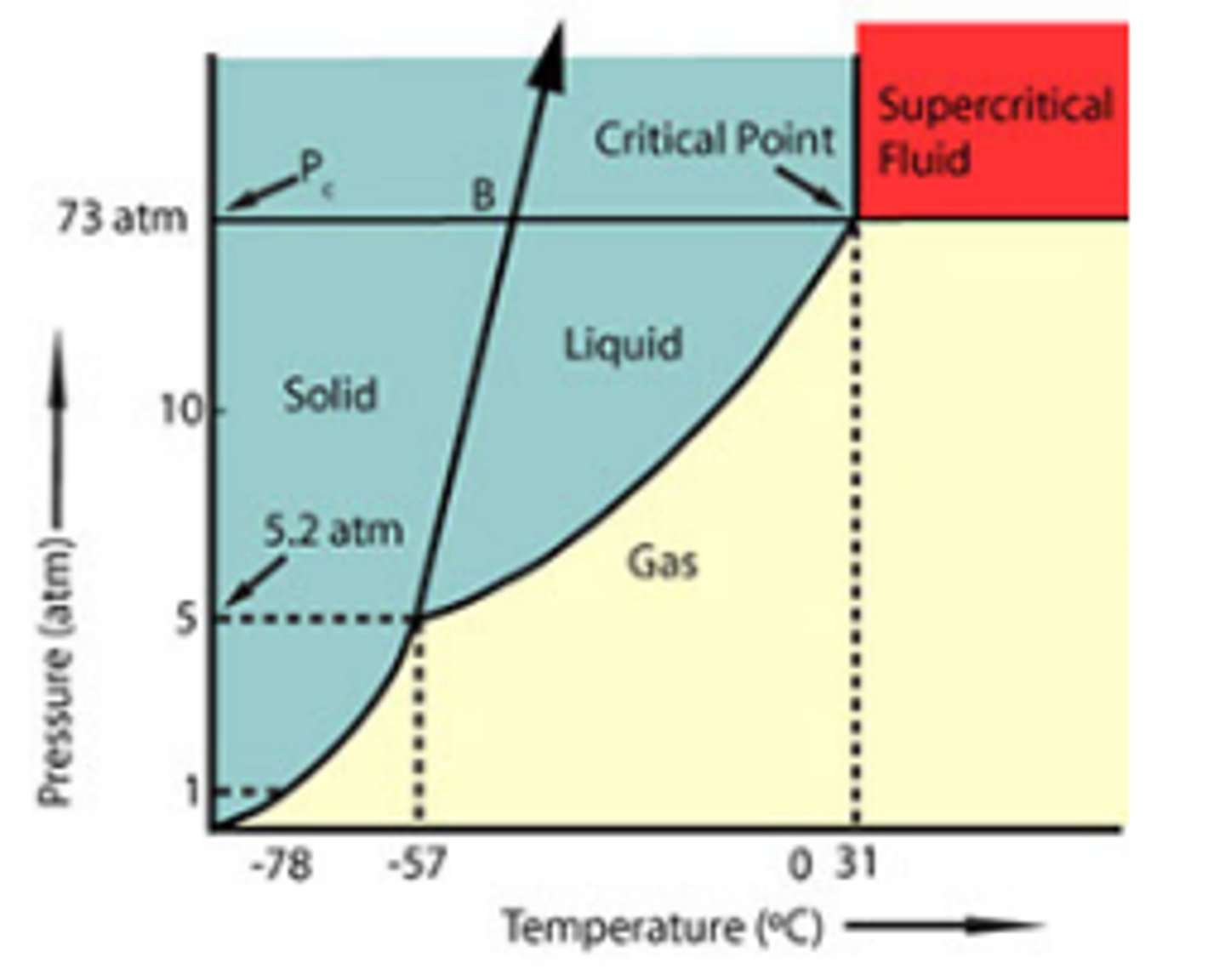
Critical Temperature
the temperature above which a substance cannot exist in the liquid state
**no pressure can liquify a gas beyond this temperature
Critical Pressure
the lowest pressure at which a substance can exist as a liquid at the critical temperature
highest critical temperture means
stronger IMFs
Triple Point
where gas, liquid, and solid states of matter coexist at a given temperature and pressure
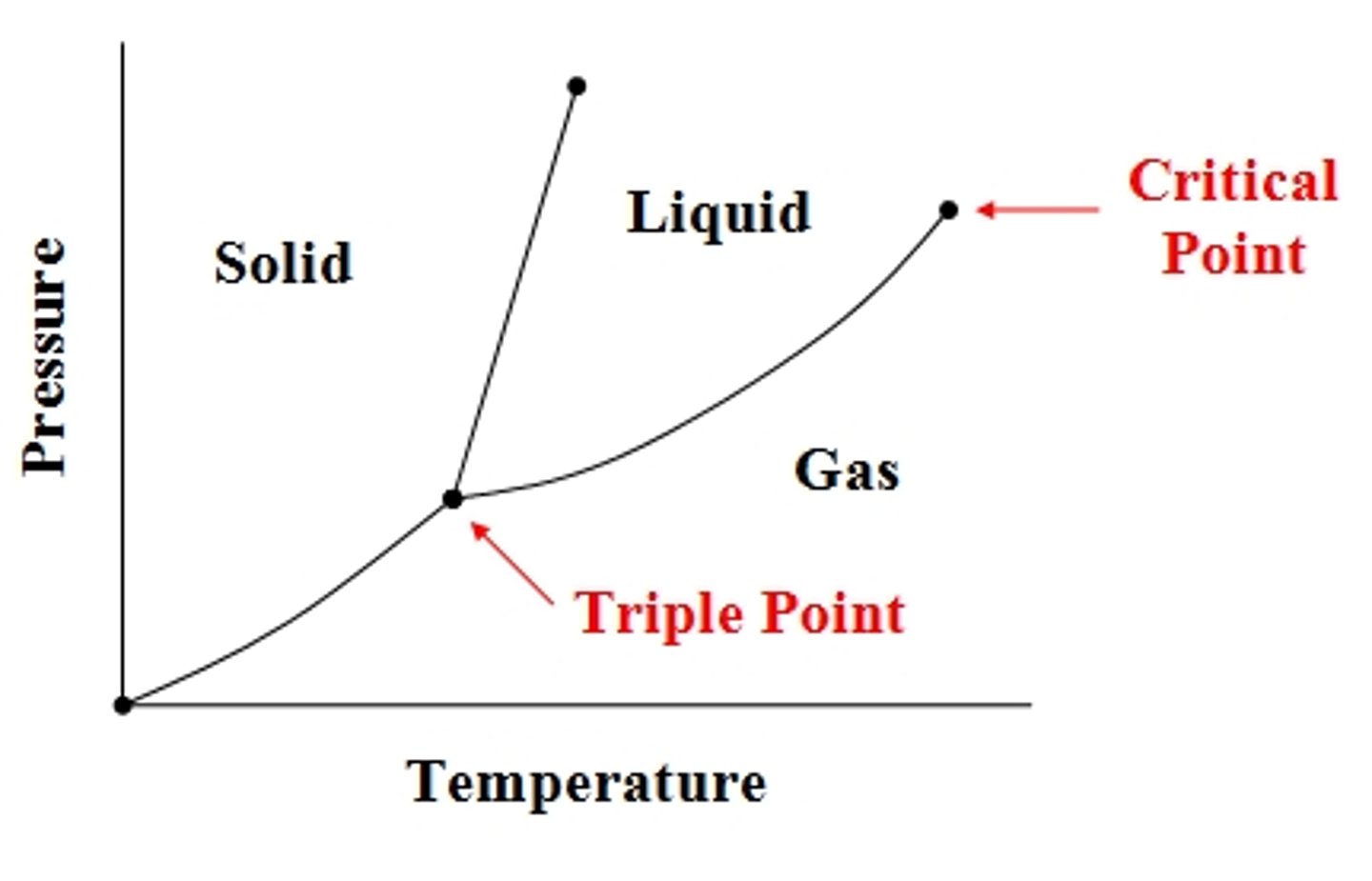
Phase Diagrams
**normal bpt/mpt is at 1 atm/760 torr