1. Atomic Mass
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
what machine is used to obtain atomic mass values
mass spectrometer
general overview of what occured during mass spectrometer simulation
- particles get seperated based on mass by a magnetic field
- since a magnetic feild has only a certain force it can apply to push the particles > the lighter the object - the more it gets deflected (moves farther), the heavier the object - the less it deflects
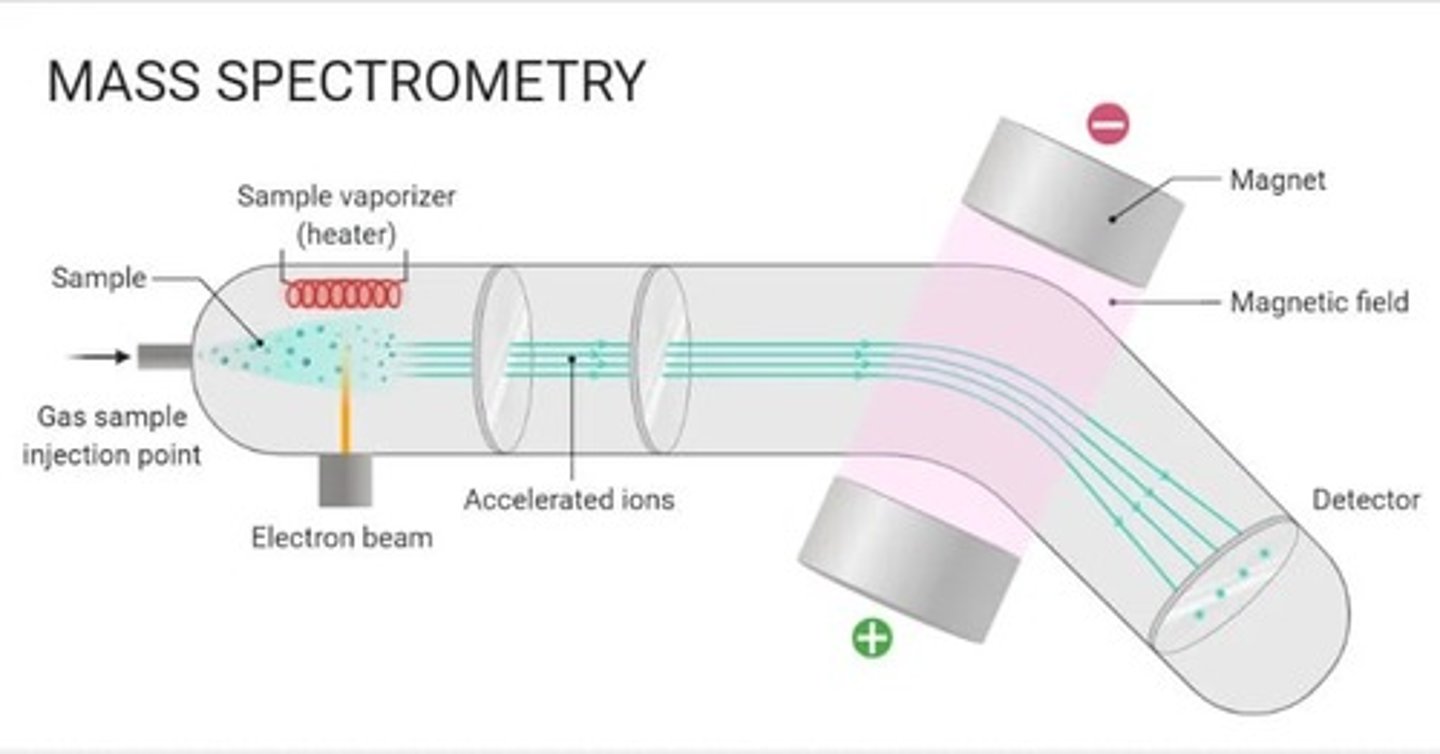
mass spectrometer labelled diagram
parts:
- sample enters
- heater that vaporizes sample (heats it up into gas)
- electron beam source from the bottom
- 2 electric plates (first one (+), second (-)
- magnet with magnetic feild
- detector at bottom
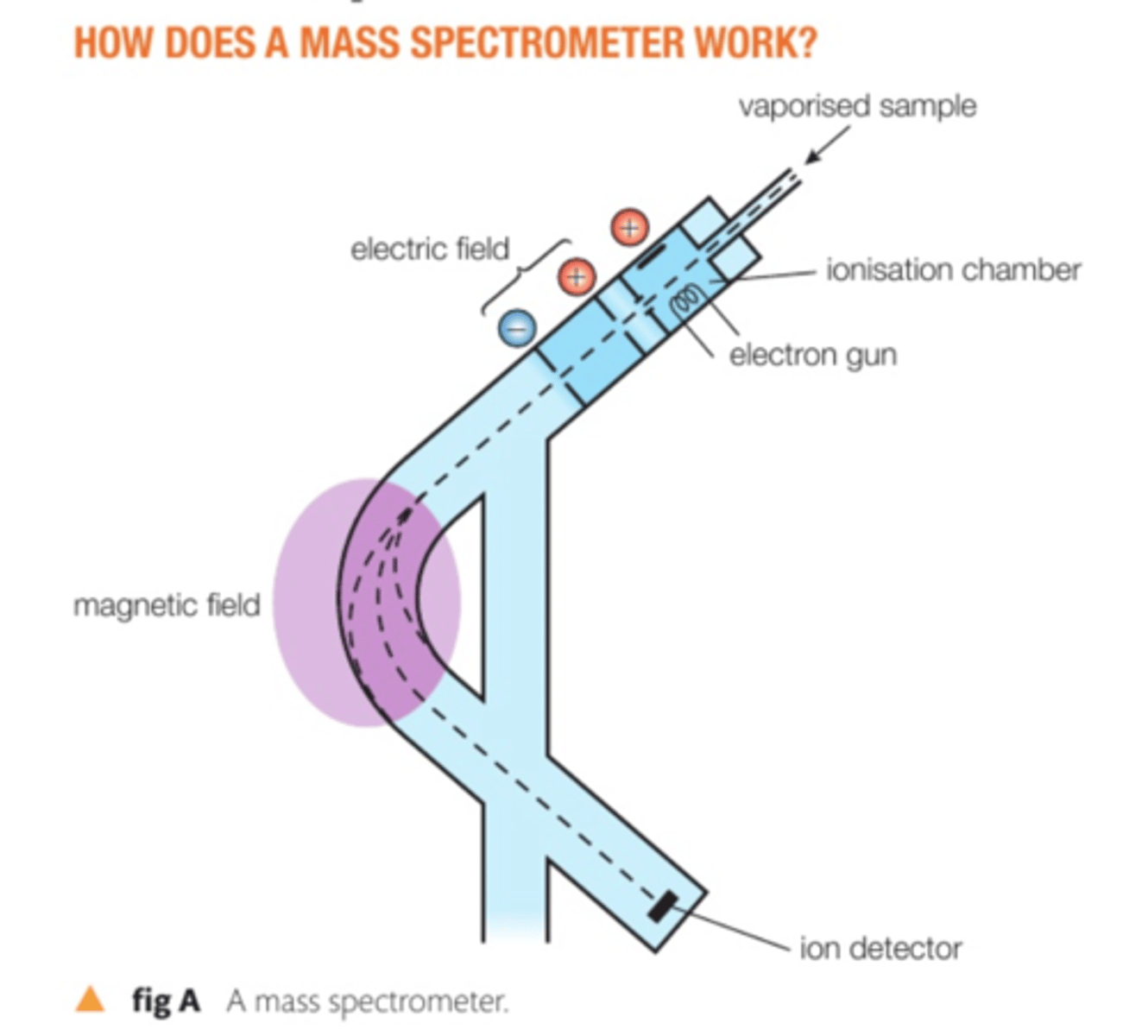
process of mass spectrometer
Vaporisation - sample enters chamber > gets heated into gas state so particles can pass easily thru rest of machine
Ionisation - sample is hit by e- beam source which knocks off some e-'s > sample is now a postively charged ion
Acceleration - the charged particles move between 2 electric plates, where they are repelled by first (+) plate and attracted to second (-) plate > so they speed up
Separation - fast moving particles then hit magnetic feild where they get deflected
Detection - deflected particles land on detection plate
what 2 things does a mass spectrometer detect + how
1) the magnetic field deflects particles depending on their mass, with lightest particles hitting furthest on detection plate and heaviest particles hitting closest to detection plate = placement determines mass
2) detector is coated in phosphorescent aterial that lights up to show where particles hit it >> determines how many particles landed in a certain location which indicates the abundance of a specifc mass of the particle
how are the results from mass spectrometer presented
on a mass spectrograph or mass spectrum
> abundance is on y-axis
> mass-to-charge ratio is on x-axis but usually the mass can be read straight from x-axis bcs only 1e- is lost per particle (bcs it gets hard to remove 2nd e- once particle is (+))
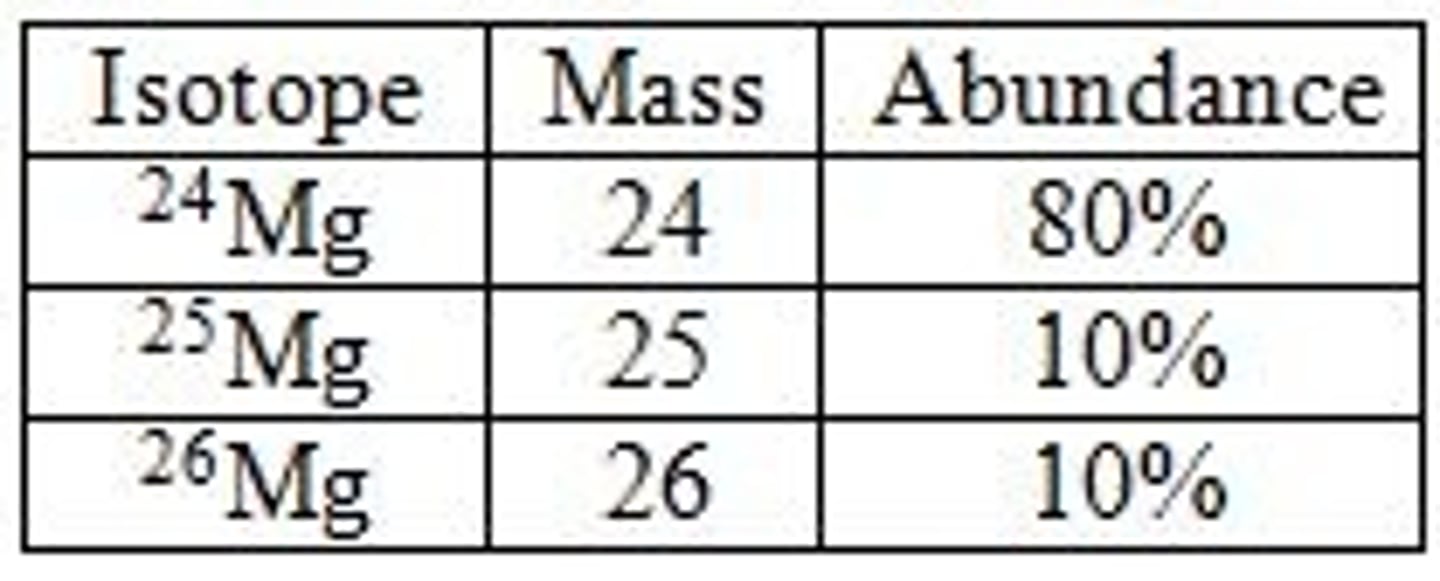
why do masses on periodic table have decimals
atoms have whole # p+ and n so their masses would be whole #s but on PT, they are decimals bcs the values are the weighted averages of all isotopes of an element
formula to find weighted average mass of an element + how many sig digits
AVG = ∑ (abundance) x (mass of isotope)
∑ = means to sum
abundance is always in the formula as a decimal
ex: if C-12 is 98.89% abundant and C-13 is 1.11% abundant, avg atomic mass of C?
Avg = (0.9889)(12.000) + (0.0111)(13.000)
= 12.011 amu
(amu = atomic mass units)
~ just do 5 sig digits for mass and 4 sig digits for abundance
if Boron's avg mass is 10.81 amu and its 2isotopes: B-10 has mass of 10.01amu and B-11 has mass of 11.01amu, determine abundance of both isotopes
AVG = ∑ (abundance) x (mass of isotope)
10.81 = (x)(10.01) + (1-x)(11.01)
^note: we put 1-x as abundance of 2nd isotope bcs 1 represents 100% - first isotope's abundance
10.81 = 10.01x + 11.01 -11.01x
11.01x - 10.01x = 11.01 -10.81
x = 0.2
so abundance of B-10 is 20% and B-11 is 80%
APP: if carbon has 3 isotopes, before even doing calcs, how can you know which is the most abundant
carbon 12 is most abundant bcs atomic mass listed on the PT is the avg of all isotopes > avg of 12 reflects the most abundant isotope's mass