Thermodynamics: Energy Transfer and Analysis
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
macroscopic forms of energy
those a system processes as a while with respect to some outside reference frame
kinetic and potential
microscopic forms of energy
those related to the molecular structure of a system and the degree of the molecular activity
internal energy (U)
the sum of all the microscopic forms of energy
kinetic energy
the energy a system possesses as a result of its motion relative to some reference frame
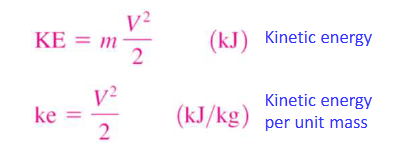
potential energy
the energy as system possesses as a result of its elevation in a gravitational field
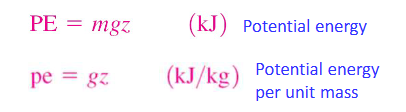
total energy
sum of all internal, kinetic, and potential energy
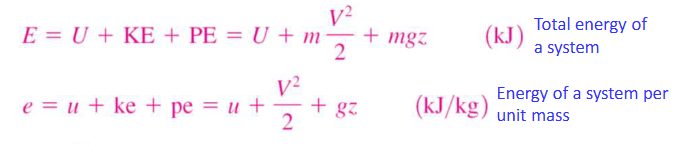
mass flow rate of a flowing fluid
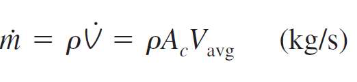
what does it mean when a variable has a dot above it
the rate of change of that variable over time (derivative)
ex. w-dot is the work done per unit time
energy flow rate of a fluid
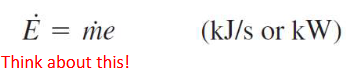
sensible energy
portion of the internal energy of a system associated with the kinetic energies of the molecules
latent energy
the internal energy associated with the phase of a system (solid, liquid, gas)
chemical energy
internal energy associated with chemical bonds
nuclear energy
internal energy associated with the strong bonds within the atom’s nucleus
thermal vs internal energy

static/organized energy
the total energy of a system, can be contained or stored in a system
dynamic/disorganized energy
forms of energy not stored in a system
recognized at the system boundary as they cross it, and they represent the energy gained or lost by a system during a process
energy transfer
energy can cross the boundary of a system in two distinct forms: heat and work
formal sign conventions for energy transfer
positive: heat transfer to a system and work done by a system
negative: heat transfer from a system and work done on a system
energy transfer for an open system
energy can also cross the boundary of a system as it is carried by the flowing mass
organized and disorganized energy conversion
we can completely convert organized energy to disorganized, but cannot completely convert disorganized energy into organized
mechanical energy
the form of energy that can be converted to mechanical work completely and directly by an ideal mechanical device
familiar forms kinetic and potential
the change in rate of mechanical energy of a fluid during incompressible flow (eqn)
mass flow rate * (pressure/density, kinetic energy, potential energy)
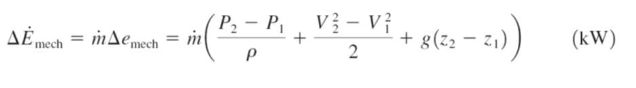
the change in mechanical energy for a fluid during incompressible flow per unit mass
pressure/density, kinetic energy, potential energy
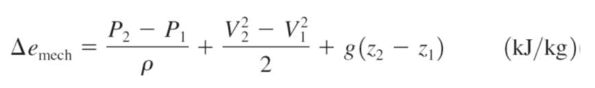
rule for pressure
pressure is not a form of energy, but it has the ability to do work
can fluid flow from low to high pressure
yes, as long as the system has a shape and configuration that allows either KE or PE to stay constant and the other to decrease
heat
the form of energy that is transferred between two systems (or a system and its surroundings) by virtue of a temperature difference
rate of energy transfer depends on the temperature difference
heat transfer per unit mass
q = Q/m (kJ/kg)
amount of heat transfer with constant rate
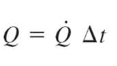
amount of heat transfer when the rate is changing
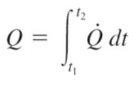
adiabatic process
no heat or matter is transferred during a process (Q = 0)
not the same as isothermal, internal temperature can still change
conduction
The transfer of energy from more energetic particles to adjacent less energetic ones due to particle interaction
convection
the transfer of energy between a solid surface and the adjacent moving fluid, involving the combined effects of conduction and fluid motion
radiation
the transfer of energy through electromagnetic waves or photons
work
all energy interaction not caused by a change in temperature
work done per unit mass
w = W/m
heat and work
both are recognized as they cross the boundary of a system (boundary phenomena)
systems process energy, but not heat or work. they are associated with a process not a system
both magnitudes depend on the system’s path, initial, and final point (path functions)