3) Acids, Bases, Buffers
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
what is pH
negative log of the activity of H+ ions in solutions
pH is never concentration, always activity
details on neutral pH
change in T&P make neutral pH change
when pH is neutral, {H+} = {OH-}
dissociation of water
Kw = {H+}*{OH-} / {H2O} = 10^-14
assume {H2O} = 1, write it out first, state assumption, then cross off
{H+} = {H2O}*Kw / {OH-}
what is pka, pkb, pkw
pka: acid dissociation constant, giving an H+
pkb: base dissociation constant, giving an OH-
pkw: p=log, logKw = log(10^-14), pkw = 14
Ka for strong acid, HCl
Ka = {H+}*{Cl-} / {HCl(aq)} = >1 because very much going to pdts
ka for weak acid, acetic acid
Ka = {CH3COO-}*{H+} / {CH3COOH} = 1.8×10^-6, much less than 1
pka = -log(1.8×10^-6) = 5.74
Kb for weak base, ammonia
kb = {NH4}*{OH-} / {NH3}*{H2O} = 1.8×10^-6
pkb = -log(1.8×10^-6) = 4.74
pka = 14-4.74=9.26
what is threshold for strong acids/bases
pka/pkb <2 is strong
lower pka means?
stronger acid
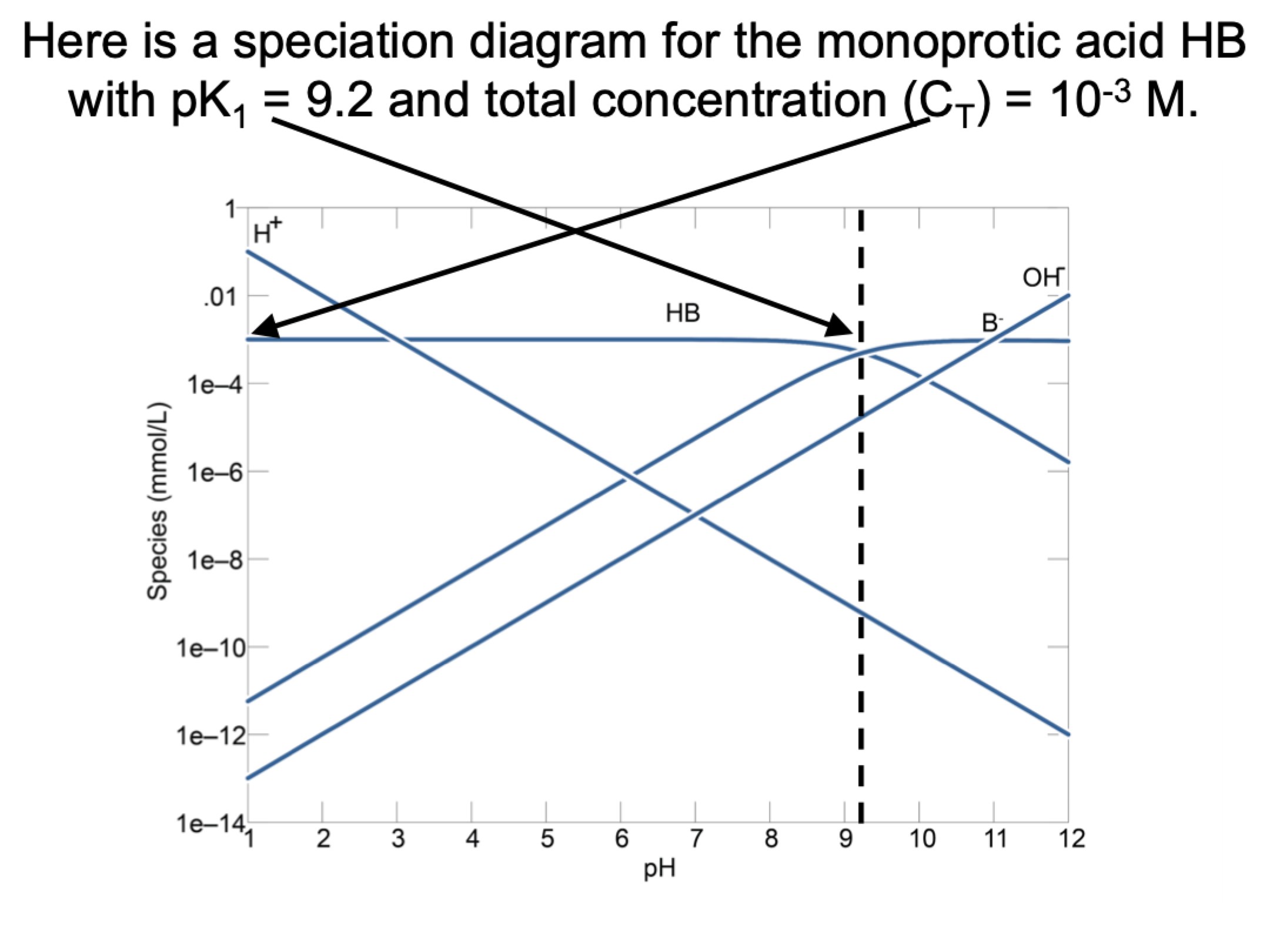
explain this graph
total conc is 10^-3
H+ line is just plotting pH (10^-1 (y) = 1(x))
the OH- line is pkw-pH
dashed line is pk1, 9.2. going from HB to B-, HB=B-, after line HB decreases at slope of 1 and B- is constant
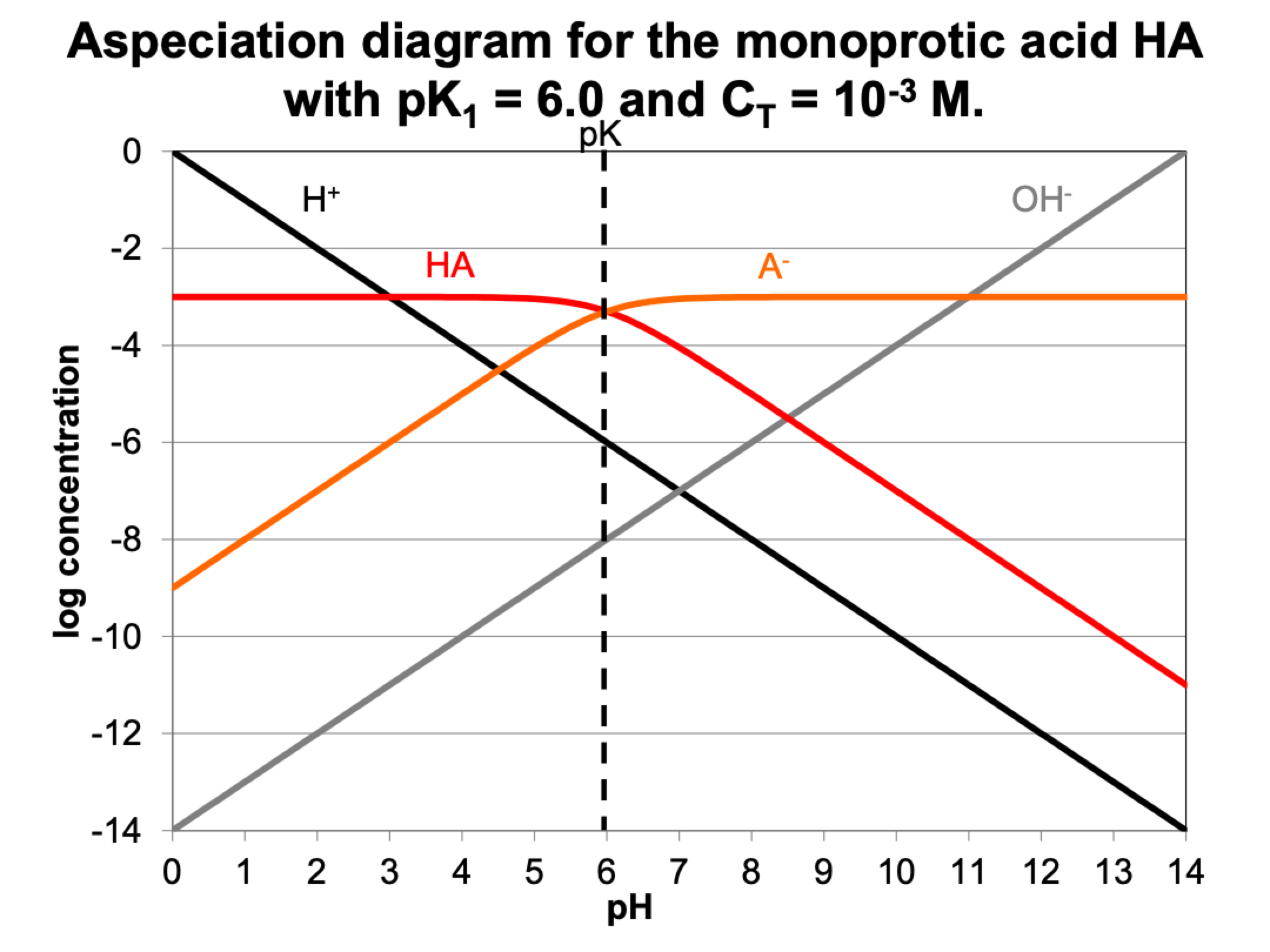
go through this example remove this card
HA <-> H+ + A-
Ka = {H+}*{A-}/HA
what are ways we can determine speciation?
speciation is how much of each ion is in solution
can determine with pH and Ka, measure CT, can calculate from that
or can look at graph, for certain pH (4), most will be HA, and very little will be A-
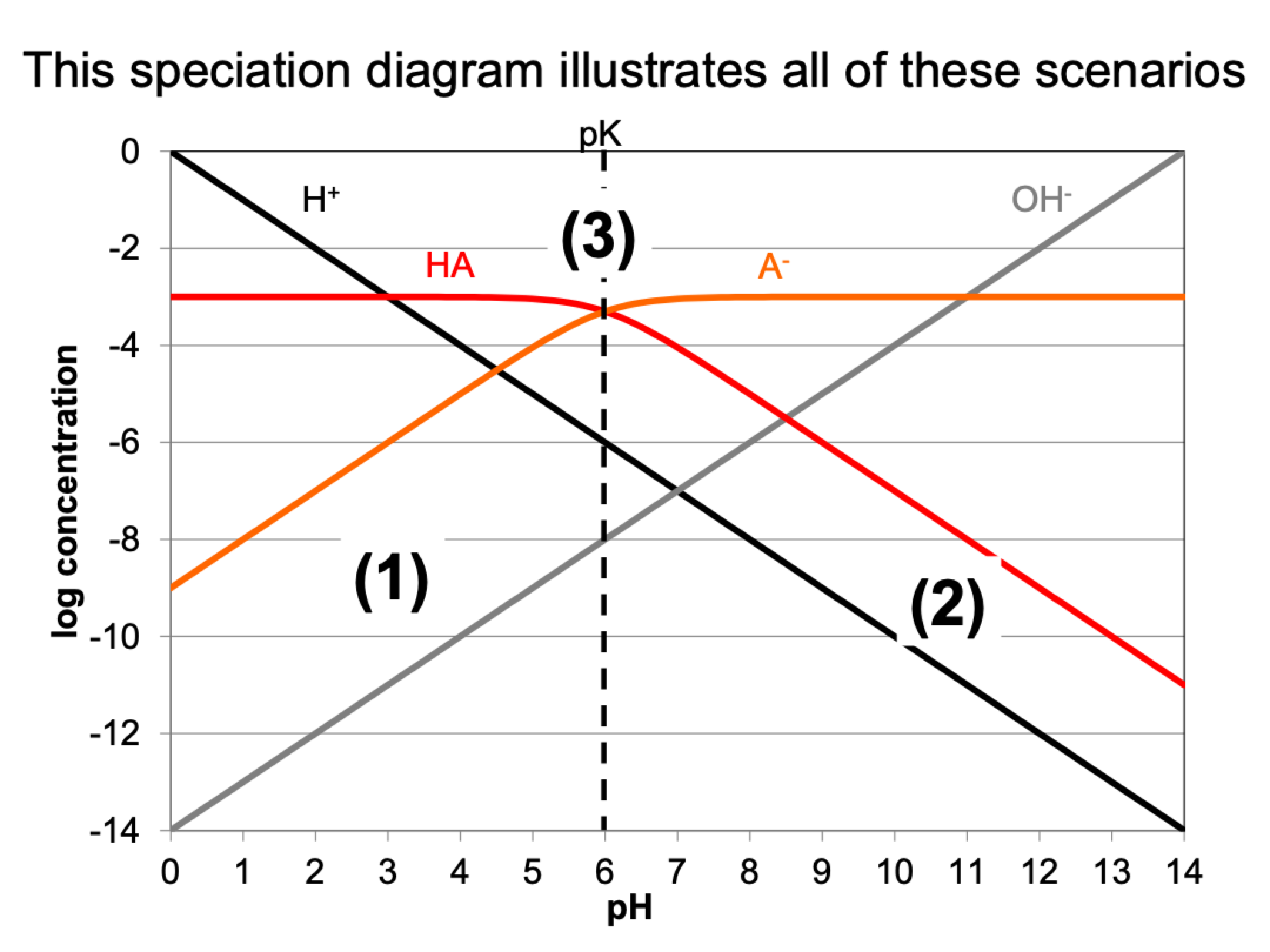
what happens at these scenarios
1: pH < pk, {H+} > K, (H+ + K = H+), HA
2: pH > pk, {H+} < K, (H+ + K = K), A-
3: pH = pk, H+ = K, H+ + K = 2H+
what is the proton balance condition
combined concentration of species that have donated protons must equal those that have accepted protons
example monoprotic acid HA
what is the pH of 10^-3 M HA solution
HA
H+ = A- + OH-
accepted = donated
accepted, HA can’t accept, so just put water accepting as H+
donated, HA can donate, and water as OH-
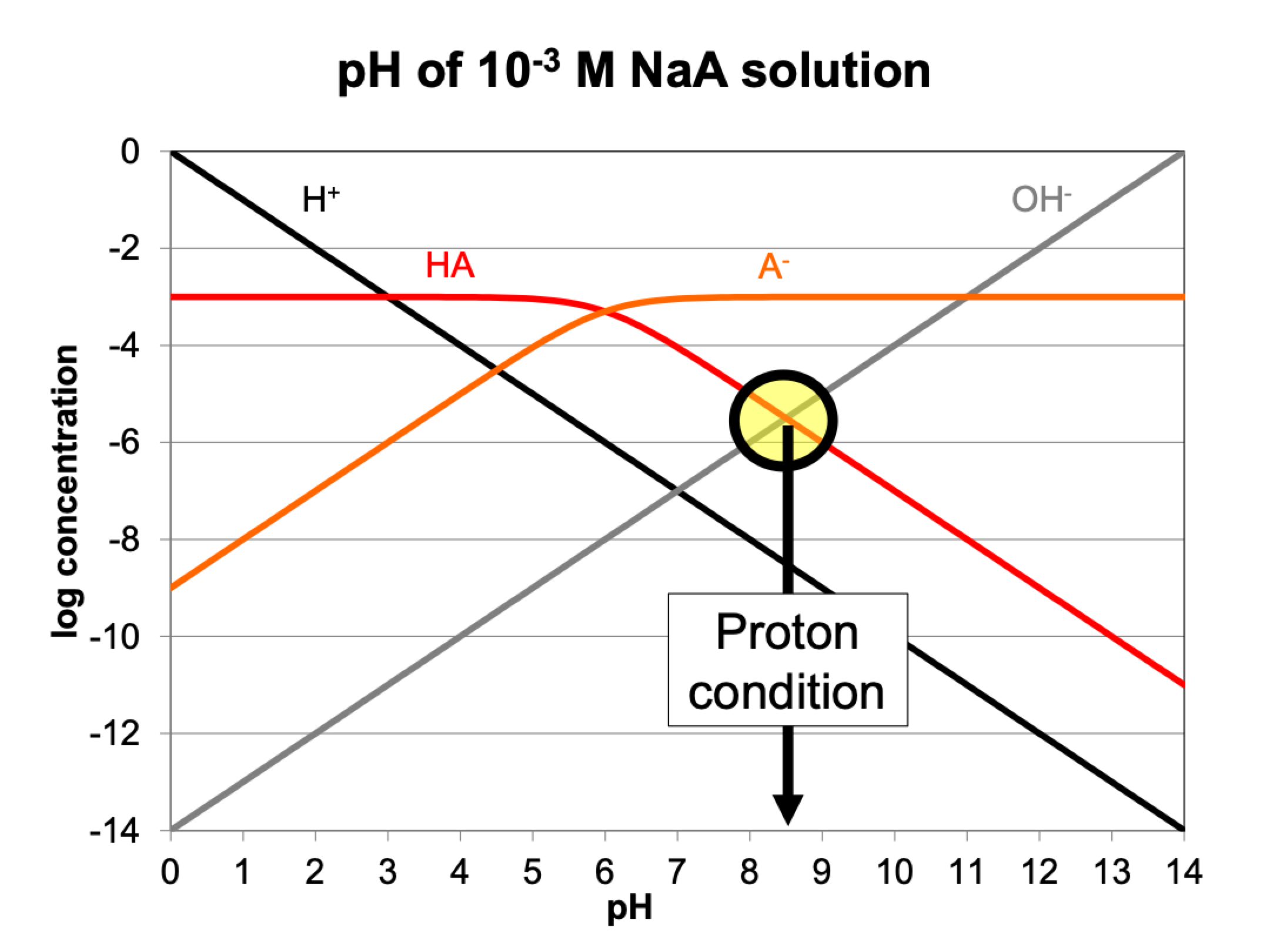
proton condition on graph (NaA)
where H+ and HA must equal OH-
Can’t be HA/A, its Ka
Can’t be A-/H+, it’s acid field and you’ve added Na
Can’t be H+/OH-, its neutral pH
Must be HA/OH-
H+ doesn’t matter in this case because where it crosses is so much lower than the other things in solution (it’s a log scale)
Only focus on the first things to cross from top down.
what are buffers
a solution capable of neutralizing both acids and bases with only small change in the pH of the system
chemistry of buffers
H+ + Ac- <-> HAc
HAc + OH- <-> Ac- + H2O
When Ac- is acting as HAc, it’s buffering
adding base or acid will buffer
When will pka = pH? (acids)
when activity of Ac- = activity of HAc
true for all acids
what is the effect of dilution on pH?
No effect
It dilutes Ac- and HAc equally, same for evaporation
Buffered solution prevents pH from changing due to dilution/evaporation
Only true for buffered solutions, and starting with equimolar concs of Ac- and HAc
where is buffering intensity highest?
at the pkas of the acid