Texture, Classification and Phase Rules in Metamorphic Rocks
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Classification of Metamorphic Rocks
metamorphic rocks are classified on the basis of texture and composition (either mineralogical or chemical)
unlike igneous rocks, their names are simple and flexible
may coose some prefx-type modifiers to attach to names
Foliated Metamorphic Rocks
Foliation: any planar fabric element
lineation: any linear fabric elements
they have no genetic connotations
some high-strain rocks may be foliated, but they are treated separately
Cleavage
traditionally: the property of a rock to split along a regular set of sub-parallel, closely-spaces planes
a more general concept adopted by some geologists is to consider cleavage to be any type of foliation in which the aligned play phyllosilicates are too fine grained to see individually with the unaided eye.
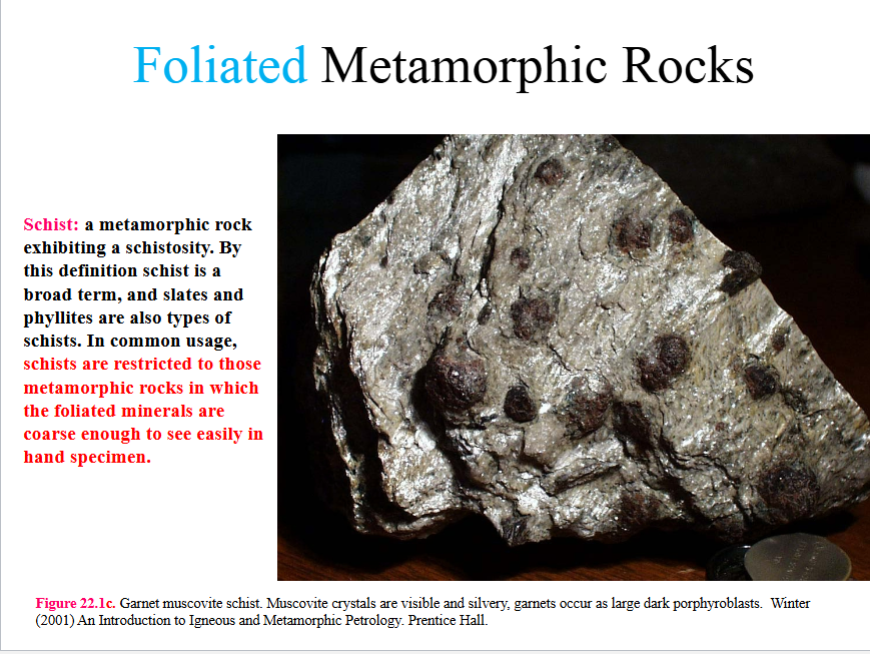
Schistosity
a preferred orientation of inequaint mineral grains or grain aggregates produced by metamorphic processes
aligned minerals are course grained enough to see with unaided eye
the orientation is generally planar, but linear orientations are not excluded
Gneissose structure
either a poorly-developed schistosity or segregated into layers by metamorphic processes
gneissos rocks are generally coarse grained

Slate vs Phyllite
Slate: compact, very fine-grained, metamorphic rock with a well-developed cleavage. Freshly cleaved surfaces are dull
Phyllite: a rock with a schistosity in which very fine phyllosilicates (sericite/phengite and/or chlorite), although rarely coarse enough to see unaided, impart a silky sheen to the foliation surface. Phyllites with both a foliation and lineation are very common

Non-Foliated Metamorphic Rocks
simpler than foliated ones
this discussion and classification applies only to rocks that are not produced by high-strain metamorphism
Granofels:
Granofels: a comprehensive term for any isotropic rock (a rock with no preferred orientation, granofelsic texture)
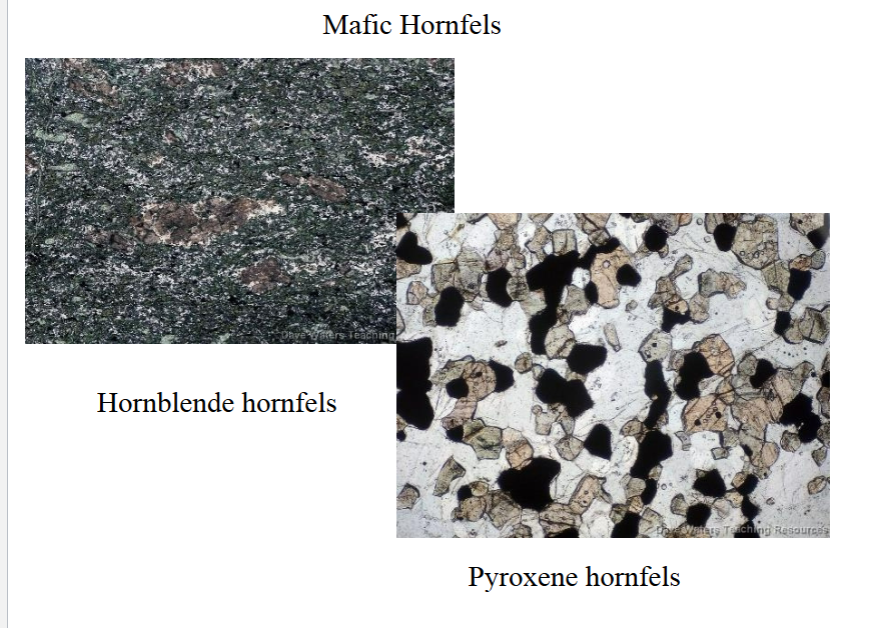
Hornfels
Hornfels: is a type of granofels that is typically very fine-grained and compact, and occurs in contact aureoles. Hornfelses are tough
Marble
metamorphic rock composed of predominately calcite or dolomite. The protolith is typically limestone or dolostone
quartzite
a metamorphic rock composed of quartz. the protolith is typically sandstone.Some confusion may result from the use of this term in sedimentary petrology for a pure quartz sandstone.
Skarn
a contact metamorphosed and silica metasomatized carbonate rock containing calc-silicate minerals, such as grossular, epidote, tremolite, vesuviantie, ect. Tactite is a synonym
Contact Metamorphism
Adjacent to igneous intrusions
• Thermal (± metasomatic) effects of hot magma
intruding cooler shallow rocks
• Occurs over a wide range of pressures, including
very low
• Contact aureole
Greenschist/Greenstone:
a low-grade metamorphic rock that typically contains chlorite, actinolite, epidote, and albite. The first three minerals are green.
Greenschist if foliated, greenstone if not.
Protolith is either mafic igneous rock or graywacke (dirty sandstone with higher proportion of feldspars, volcanic rock fragments, silt and clay)
Amphibolite
metamorphic rock dominated by hornblende + plagioclase. Amphibolite may be foliated or non-foliated. The protolith is either a mafic igneous rock or graywacke
Blueschist
A blue amphibole (glaucophane)- bearing metamorphosed mafic igneous rock or mafic graywacke. This term is so commonly applied to such rocks that is is even applied to non-schistose rocks
Eclogite
a green and red metamorphic rock that contains clinopyroxene and garnet (omphacite + pyrope). the protolith is typically basaltic
Granulite
a high grade rock of pelitic, mafic, or quartzo-feldspathic parentage that is predominately composed of OH-free minerals. Muscovite is absent and plagioclase and orthopyroxene are common
migmatiite
a composite silicate rock that is heterogeneous on the 1-10 cm scale, commonly having a dark gneissic matrix (melansome) and lighter felsic portions (leucosome). Migmatites may appear layered, or the leucosomes may occur as pods or form a network of cross-cutting veins
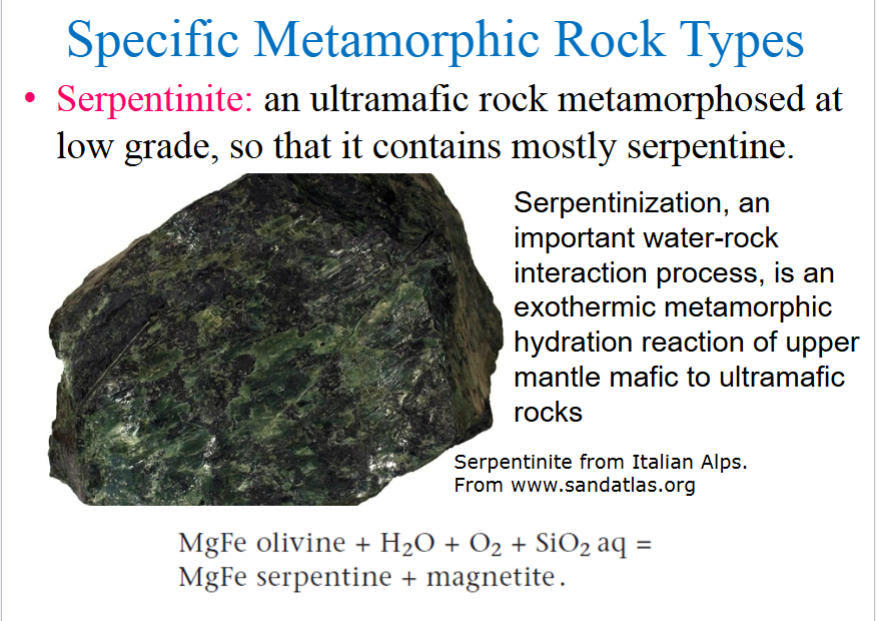
Serpentinite
an ultramafic rock metamorphosed at low grade, so that it contains mostly serpentine
Serpentinization, an important water-rock interaction process, is an exothermic metamorphic hydration reaction of upper mantle mafic to ultramafic rocks
Additional Modifying Terms: Porphyroblastic
Porphyroblastic means that a metamorphic rock has one or more metamorphic minerals that grew much larger than the others. Each individual crystal is a porphyroblast
Some porphyroblasrs, particularly in low-grade, contact metamorphism, occur as ovoid “spots”
If such spots occur in a hornfels or a phyllite (typically as a contact metamorphic overprint over a regionally developed phyllite), the terms spotted hornfels, or spotted phyllite would be appropriate
auge
Some gneisses have large eye-shaped grains
(commonly feldspar) that are derived from pre-
existing large crystals by shear (as described in
Section 23.1). Individual grains of this sort are
called auge (German for eye), and the (German)
plural is augen. An augen gneiss is a gneiss with
augen structure (Fig. 23-18).

Additional Modifying Terms: Ortho and Para
Ortho- a prefix indicating an igneous parent, and
Para- a prefix indicating a sedimentary parent
The terms are used only when they serve to dissipate doubt. For example, many quartzo-feldspathic gneisses could easily be derived from either an impure arkose or a granitoid rock. If some mineralogical, chemical, or field- derived clue permits the distinction, terms such as orthogneiss, paragneiss, or orthoamphibolite may be useful
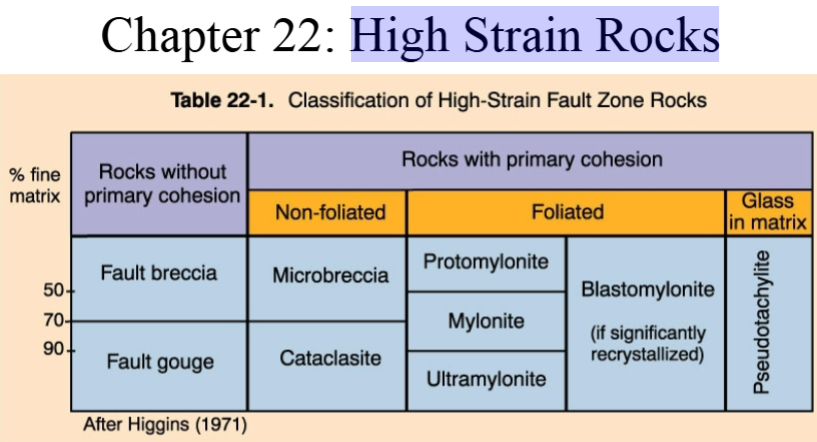
High Strain Rocks
The Phase Rule in Metamorphic Systems
If F greater than or equal to 2 is the most common situation, then the phase rule may be adjusted accordingly:
F = C - P + 2 Greater than or equal to 2
P less than or equal to C (Eq 24.1)
Goldschmidt’s mineralogical phase rule, or simply the mineralogical phase rule
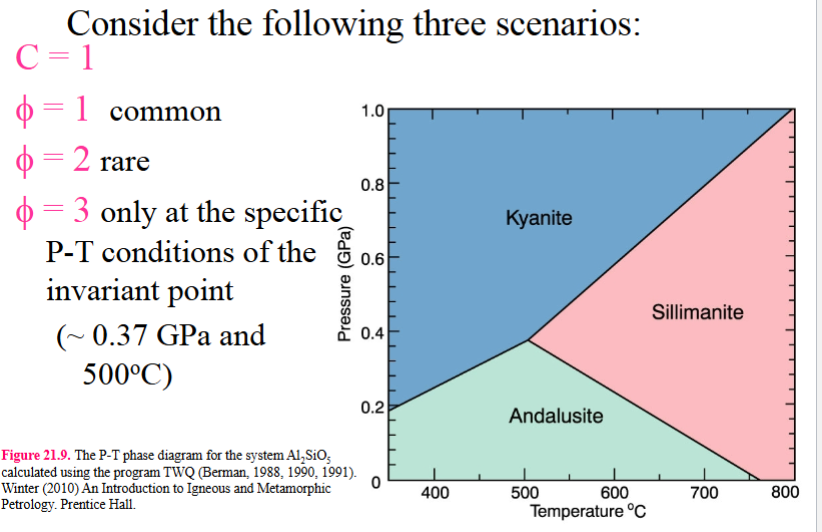
Consider the following three scenarios:
C=1
P=1 common
P=2 rare
P= 3 only at the specfic P=T conditions of the invarient point (0.37 GPa AND 500 c)
Suppose we have determined C for a rock. Consider the following three scenarios:
P=c
P=C
The standard divariant situation
The rock probably represents an equilibrium mineral assemblage from within a metamorphic zone
P<C
Common within mineral systems that exhibit solid solution
P>C
A more interesting situation, and at least one of three situations must be responsible
F<2
The sample is collected from a location right on a univariant reaction curve (isograd) or invariant point
Equilibrium has not been attained
The phase rule applies only to systems at equilibrium, and there could be any number of minerals coexisting of equilibrium is not attained.
We didnt choose the # of components correctly
Some guidelines for an appropriate choice of C
Begin with a 1-component system, such as CaAl2Si2O8 (anorthite), there are 3 common types of major/minor components that we can add
a. Components that generate a new phase
Adding a component such as diopside results in an additional phase: in the binary Di-An system diopside coexists with anorthite below the solidus
b. Components that substitute for other components
Adding a component such as albite to the 1-C anorthite system would dissolve in the anorthite structure, resulting in a single solid-solution mineral (plagioclase) below the solidus
Fe and Mn commonly sub for Mg
Al may sub for Si
Na may sub for K
C. Perfectly mobile elements
Mobile components are either a freely mobile fluid component or a component that dissolves readily in a fluid phase and can be transported easily
The chemical activity of such components is commonly controlled by factors external to the local rock system
They are commonly ignored in deriving C for metamorphic systems
How do you know which way is correct?
Rocks should tell you:
Phase rule= interpretive tool, not predictive
If only see low-P assemblages → some component may be mobile
if many phases in an area it is unlikely that all is right on univariant curve, and may require the number of components to include otherwise mobile phases, such as H2O or CO2, in oredr to apply the phase rule correctly
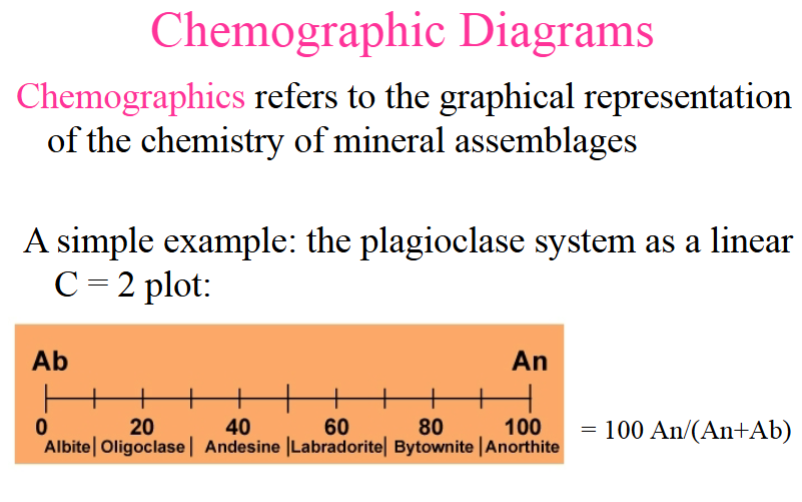
Chemographics
Chemographics refers to the graphical representation of the chemistry of mineral assemblages
A simple example: the plagioclase system as a linear c=2 PLOT:
Chemographic Diagrams
What happens if you pick a composition that falls directly on a tie-line, such as point (f)?
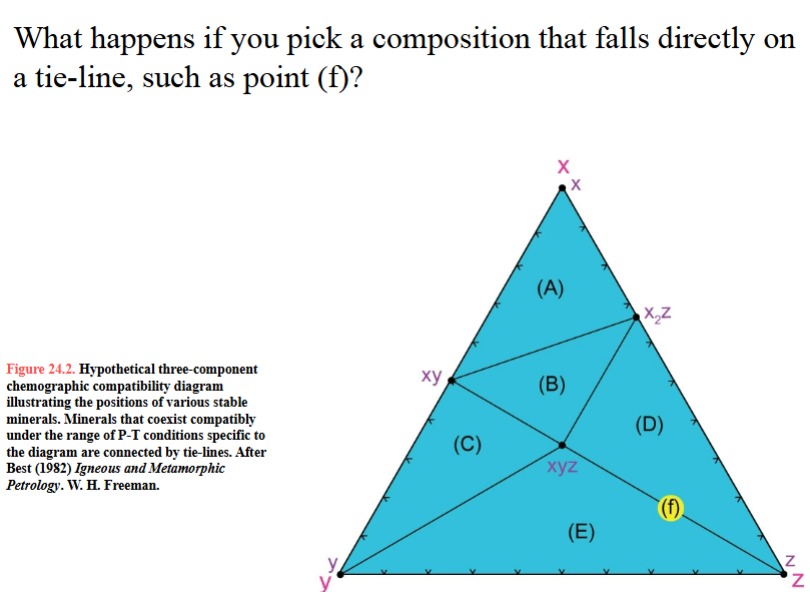
“compositionally degenerate”
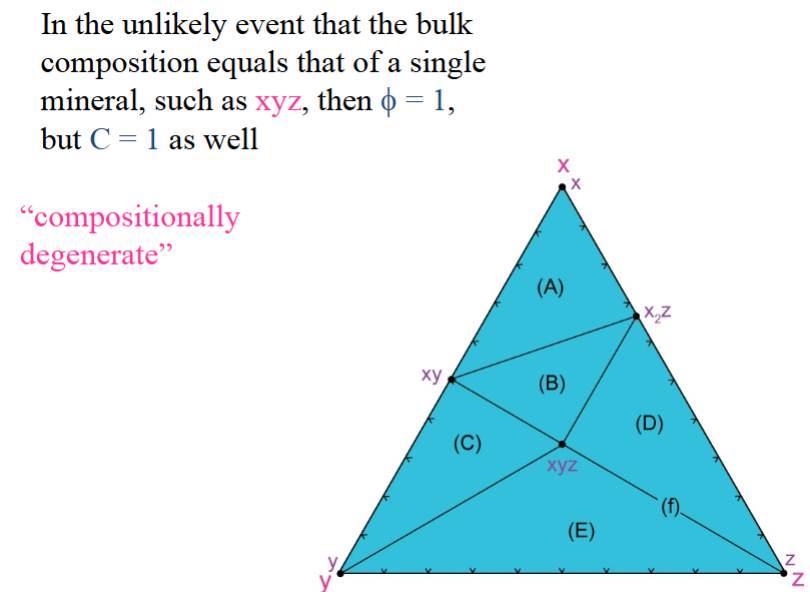
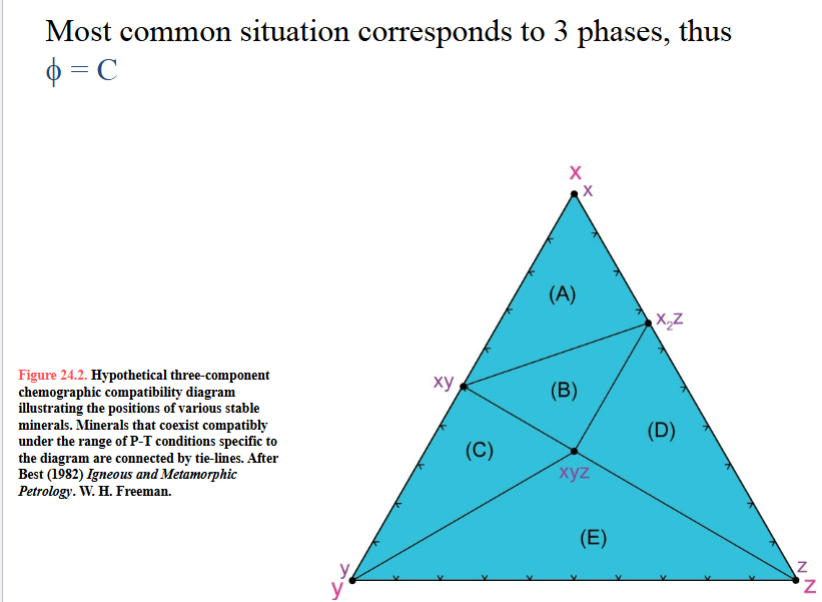
Valid compatibility diagram must be:
must be references to a specific range of P-T conditions, such as a zone in some metamorphic terrane, because the stability of the minerals and their groupings vary as P and T vary
solid solution chemograph
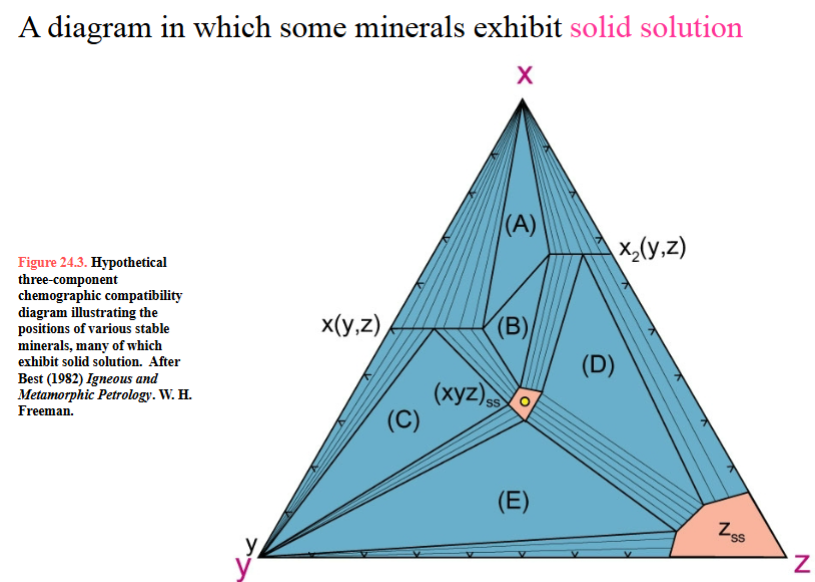
What is the “right” choice of components? How to pick
Simply “ignore” components
trace elements
elements that enter only a single phase (we can drop both the component and the phase without violating the phase rule)
perfectly mobile components
Combine components
components that sub for one another in solid solution (Fe + Mg)
Limit the types of rocks to be shown
only deal with a sub-set of rock types for which a simplified system works
use projections
ill explain this in this in the next lecture
The ACF Diagram
illustrate metamorphic mineral assemblages in mafic rocks on a simplified 3-C triangular diagram
concentrate only on the minerals that appear or disappeared during metamorphism, thus acting as indicators of metamorphic grade
The three pseudo-components are all calculated on an atomic basis:
A= Al2O3 + Fe2O -Na2O - K2O
C= CaO - 3.3P2O5
F= FeO + MgO + MnO
A= Al2O3 + Fe2O -Na2O - K2O Why the subduction?
Na and K in the average mafic rock are typically combined with Al to produce Kfs and Albite
in the ACF diagram, we are interested only in the other K-bearing metamorphic minerals, and thus only in the amount of Al2O3 that occurs in excess of that combined with Na2O and K2o (in albite and K-feldspar)
Bc the ratio of Al2O3 to Na2O or K2O in feldspars is 1:1, we subtract from Al2O3 an amount equivalent to Na2O and K2O in the same 1:1 ratio
The AKF Diagram
because pelitic sediments are high in Al2O3 and K2O, and low in CaO , Eskola proposed a different diagram that included K2O to depict the mineral assmblages that develop in them
Three of the most common minerals in metapelites: andalusite, muscovite, and microcline, al plot as distinct points in the AFK diagram
In the AFK diagram, the pseudo-components are
In the AFK diagram, the pseudo-components are
A= Al2O3 + Fe2O3 - Na2O -K2O - CaO
K= K2O
F = FeO +MgO +MnO