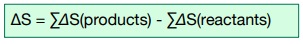Entropy
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What is entropy
The measure of the disorder of a system
Factors affecting the entropy of a system
Physical state
Gases>Liquid>Solids
Temperature
Temperature ∝ Entropy
Number of Particles
N2O4—→ 2NO2
The more particles in a system the higher the entropy. eg there are more ways to arrange 10 particles ie permutations than 2 particles.
Pressure/Volume
Increasing the volume or decreasing the pressure increases entropy
Complexity (no of bonds)
Bonds ∝ Entropy eg Pentane has higher entropy than hydrogen because there are more bonds and those bonds can arrange themselves in different ways
Flexibility
The more flexible a molecule, the more ways the bonds can be arranged and have higher entropy eg Hex-1-ene has higher entropy than cyclohexane
Calculating Entropy