CHEM 2070 Alkanes, Cycloalkanes, IUPAC, Cyclohexanes
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
IUPAC naming
The standard for organic and inorganic compounds
International Union of Pure and Applied Chemistry
Parent chain
The longest continuous chain of carbons. The name is based off the number of carbons and suffix ‘-ane’
Substituent
Hydrogen atom or group coming off your parent chain
Methane
1 carbon
Ethane
2 carbons
Propane
3 carbons
Butane
4 carbons
Pentane
5 carbons
Hexane
6 carbons
Heptane
7 carbons
Octane
8 carbons
Nonane
9 carbons
Decane
10 carbons
How do you name rings?
Using prefix ‘cyclo-’ then the number of carbons
Steps of IUPAC naming
Identify the parent chain
Number the parent chain in a way to give substituents the lowest possible numbers
Name the substituents with the locator number
Assemble name: Locator numbers use a hyphen, no spaces between the substituents and parent chain
More than one substituent
Use the parent chain and choose the numbers that give the lowest locator numbers
If its a tie between two or more substituents?
Look at the second substituents
If the tie can’t be broken between two or more substituents?
Use alphabetical order
Naming Rule of more than one of the same substituent
Determine parent chain
Order substituents, order alphabetically (excluding the prefix)
Add multiplying prefix
Add locator number for each substituent, separated by a comma
Naming Cycloalkanes
Distinguish between ring carbons and chain carbons (parent chain)
Ring C >= alkyl C, the ring is the parent
Ring C < alkyl C, the ring is the substituent
The carbon with the most substituents (if possible), then go in direction that gives the lowest locator numbers
Naming substituents
Alkane chains coming off a parent chain can also be substituents. Naming it with the prefix from the number of carbons plus ‘-yl'
1 carbon atom in substituent
Methyl
2 carbons atom in substituent
Ethyl
3 carbons atom in substituent
Propyl
4 carbons atom in substituent
Butyl
5 carbons atom in substituent
Pentyl
6 carbons atom in substituent
Hexyl
7 carbons atom in substituent
Heptyl
8 carbons atom in substituent
Octyl
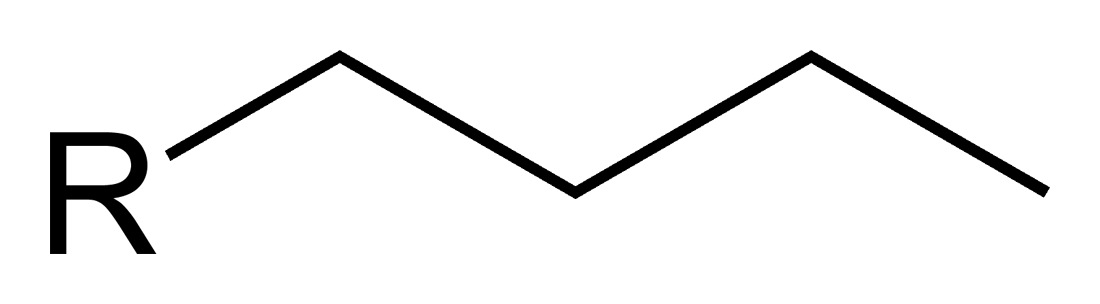
What is this?
Butyl
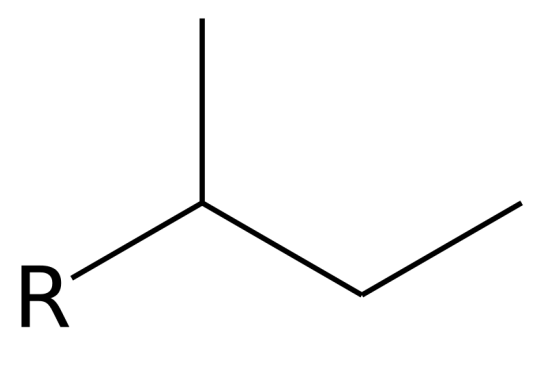
What is this?
sec-butyl
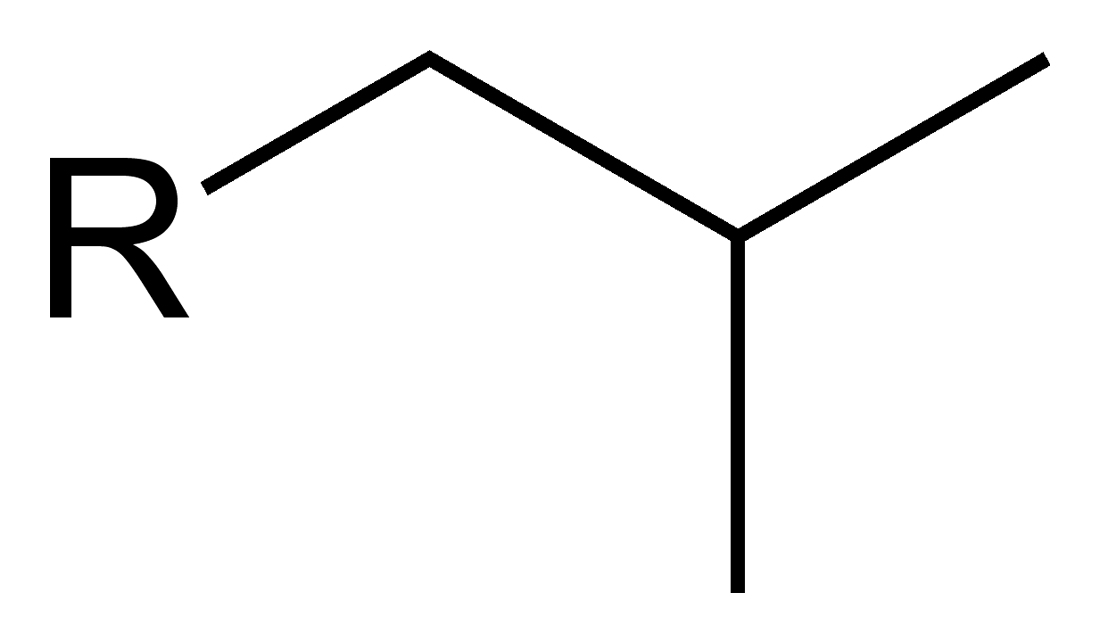
What is this?
Isobutyl
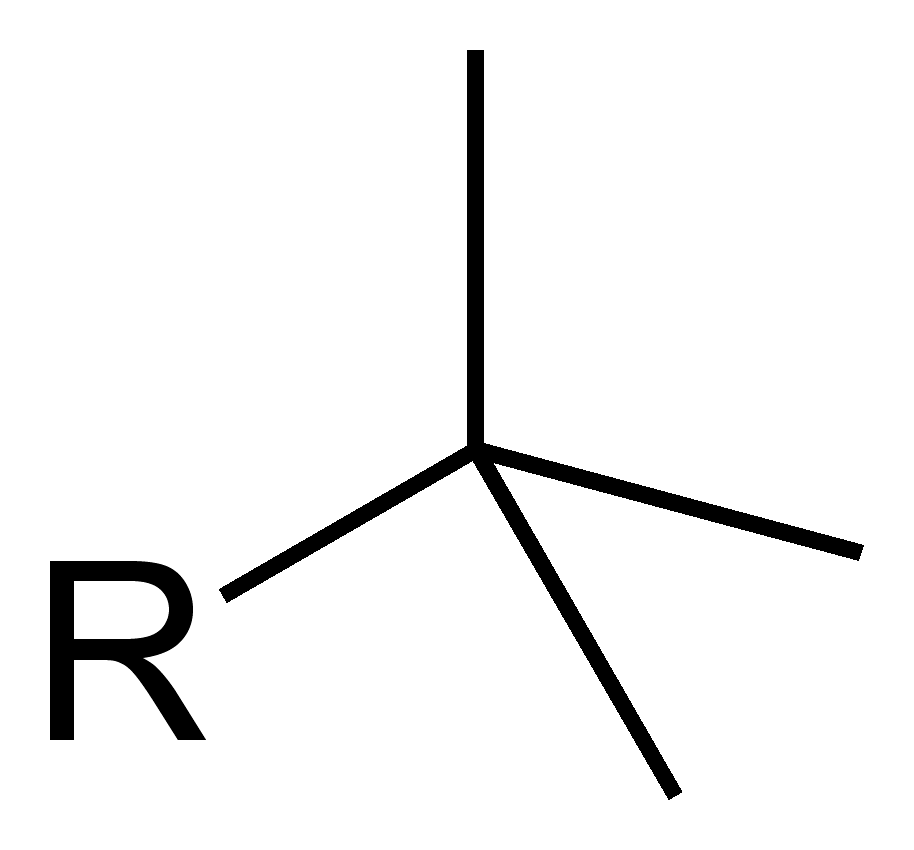
What is this?
tert-butyl
Naming Complex Branching
Name separately first
Number the longest carbon chain within the substituent. Always start with C attached to the parent chain where the complex branch is attached to the parent
Name the substituent and add the side groups. () says it’s one unit
Isomers
Having the same formula, but different structure
Constitutional isomers
Having the same structure, but different connectivity
Conformers
Single bonds that can rotate, resulting in multiple 3D shapes
Newman Projections
Compare the relative stability of possible conformations (single-bond rotations)
Dihedral angle (torsional angle)
The angle between atoms on adjacent carbons
Steric strain
When groups come together, electrons are forced to occupy the same space (repulsion)
Ideal bond angles for sp³ hybridized carbon
109.5
Angle strain
The deviation from the ideal angle because of a ring
Torsional strain
Due to the restricted rotation
Cyclopropane characteristics
3 carbons
Significant strain, energy=9.2 kJ/mol
Planar ring
Bonds are eclipsed
Cyclobutane characteristics
4 carbons
Lots of strain, a little less torsional strain
Bonds mostly eclipsed
Cyclopentane characteristics
5 carbons
Little torsional strain
“Envelope conformation”
Partly eclipsed
Cyclohexane
6 carbons
Essentially no angle strain, still no torsional strain
Axial
Being parallel to an axis, like x or y
Equatorial
Being relatively to the “equator” of a cyclohexane
What four things NOT to do when drawing chair structures?
Up axial on a down carbon (mixing ups/downs)
Wedges/dashes
Equatorial in the wrong direction (must be parallel)
Something in between axial and equatorial
1,3-diaxial interactions
Steric strain from substituents in the axial position
Dashes indicate what when drawing chair conformations?
Down
Wedges indicate what when drawing chair conformations?
Up
Bicyclic
Compounds that contain two fused rings
Bridgehead carbons
Carbons where rings are fused together
Naming bicyclic compounds
Prefix ‘bicyclo-’ + # of carbons in each piece of the rings + parent chain
Declain
Two 6-member rings fused together; found in many naturally occurring compounds