CHMA 2002 - Module 2E: Structure and function of a protein
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
The right side and systematic veins carry
oxygen poor and cO2 rich blood
Systematic arteries and the left side carry
oxygen rich and CO2 poor blood
Hb is
hemoglobin
Mb is
myoglobin
myoglobin is a __________ protein
monomeric
Myoglobin is a ___ structure
tertiary (3)
Myoglobin is conjugated because it
has a non-protein component called Heme (in red)
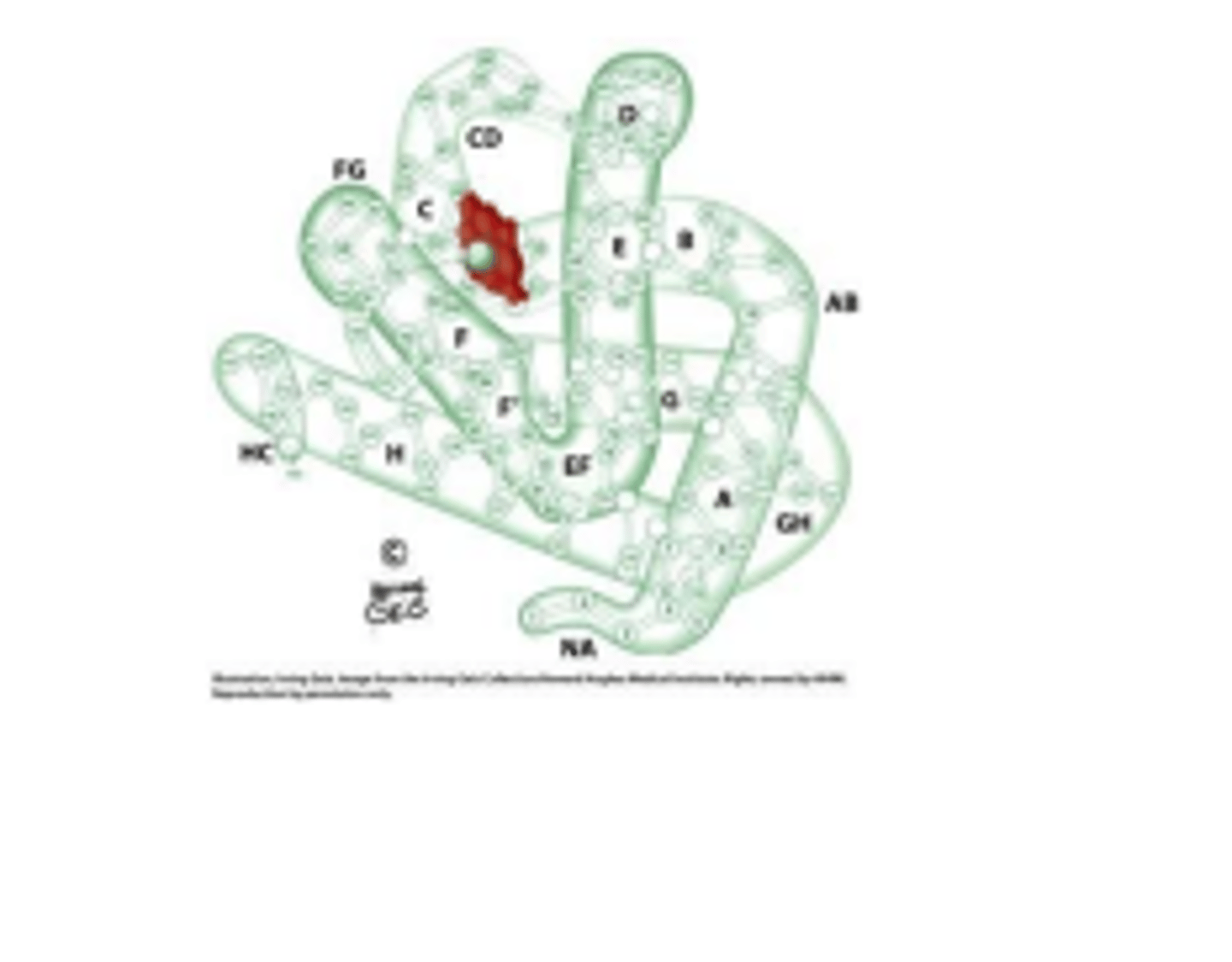
myoglobin facilitates the diffusion of oxygen from
blood to muscle
myoglobin serves to
store O2
Hemoglobin is a _____________ protein with a ___________ structure
tetrameric , quaternary
hemoglobin serves as an
O2 carrier
Hemoglobin supplies oxygen from
the lungs to Mb in muscle
both Mb and Hb can
selectively and reversibly bind O2
the stability of Mb is from
non-covalent, hydrophobic core
Between Mb and Hb, which is a carrier protein and which is a storage protein?
Hb=carrier
Mb=storage
Myoglobin is a ______________protein
monomeric
Hemoglobin is a ____________ protein
tetrameric
Mb and Hb have ____________ and ______________ binding to O2
selective and reversible
myoglobin is a bundle of
8 alpha helices
Myoglobin is rendered stable due to its
hydrophobic core
The prosthetic heme group in myoglobin is located in the
hydrophobic core between E and F helices
To reversibly bind oxygen to heme, an electron is temporarily transferred from
Fe2+ to O2
Hb has how many subunits, each of which contain a...
4, heme group
blue is
deoxy
red is
oxy
oxygenation occurs with ____________ bonding
non-covalent
What shape is the binding curve of Hb
sigmoidal
what shape is the binding curve of Mb
hyperbolic
T/F: The subunits of Hb are identical to Mb
False, similar but not identical. Mb has eight alpha helices and Hb subunits only have 7
The sigmoidal shape of binding curves in Hb indicates
binding of one O2 molecule to a heme group, facilitating faster binding of O2 to the other heme groups of Hb
The phenomenon of the sigmoidal shape of binding curves in Hb is called
positive cooperativity of binding
What is the reason for positive cooperativity of binding in Hb
Allosteric regulation:
The conformational changes occurred in the quaternary structure of Hb
When P50 is lower,
affinity to oxygen to these molecules is higher
P50 compares
binding affinities