Catalysts
1/44
Earn XP
Description and Tags
Applied Chem 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
Catalyst
A substance that increases the rate of reaction remaining itself unchanged by providing an alternative reaction pathway characteristic of lower activation energy and increased effective collisions.
Why do transition metals make such good catalysts
They change oxidation states by gaining or losing electrons within their D orbitals.
Solid Catalyst Composition
Active Site
Promoter (Textual, electrical, poison resistant)
Support/Carrier
Describe the role of the support medium
Support mediums are often used to maximise the surface area of a catalyst. They help minimise the cost of the reaction because only a small coat of catalyst is needed to provide a large surface area.
Solid Catalyst Preparation
1) Precipitation
2) Ion Exchange
3) Impregnation Method
Mechanism of Heterogeneous Catalysis
a) Adsorption of the reactant
b) Diffusion of reactants along the surface to reach the active site
c) Reaction at an active site to form adsorbed products
d) Desorption of product
Important Heterogeneous Processes
Haber- Bosch (Iron Fillings and Nickel for Steam Reforming)
Ostwald Process (Platinum-rhodium catalyst)
What is the active site
Active sites are the atoms or crystal faces where the reaction actually occurs.
Explain how heterogeneous catalysts work
Heterogeneous catalysts work by adsorbing reactants onto active sites located on their surfaces.
Where are the active sites located
At the surface of the heterogeneous catalyst
Describe the role of active sites
Reactant Adsorption
Bond activation and weakening
Orientation and Rearrangement
Product desorption
Adsorption
Physiochemical process by which an atom or molecule attaches itself onto the surface of a solid state material.
Types of Adsorption Processes
Physisorption
Chemisorption
Condensation
Desorption
Physiochemical process by which an atom or molecule detaches itself from the surface of a solid state material.
Absorbate
Gas or vapour species which adsorbs on a surface
Adsorbent
Solid substrate that provided surface for adsorption.
Hysteresis
Difference in adsorption versus desorption parts of an isotherm.
Adsorbent Characteristics
High Surface area
Proper Poore structure and size distribution
Good Mechanical strength
Thermal stability
Explain why it is not desirable to use a very effective catalyst for this reaction.
Reaction may be too fast and may become uncontrolled.
May cause an explosion.
Heterogeneous catalyst
In a different phase from reactants
What type of catalyst does the contact process use?
Heterogenous
What is the contact process?
A process that produces sulfuric acid
What catalyst is used for the contact process?
Vanadium (V) oxide V₂O₅
Describe the use of iron in the Haber process
Acts as a catalyst
Describe the effect that catalyst poisoning has on the reaction
Catalysts poisoning reduces the surface area of the catalysts available to the reactants , slowing down the reactions.
What is catalyst poisoning?
This is when the reaction mixture may also bind to the catalysts's surface and block the reactants from being adsorbed.
What economic burden does catalyst poisoning have on industries?
Catalyst poisoning increases the cost of a chemical process because less product is made in a given time. The catalyst may even need to be replaced which is expensive
Explain how heterogeneous catalysts can become poisoned
Catalysts can become poisoned if active sites are covered with impurities
Homogeneous catalyst
In the same physical state as the reactants
Describe how homogeneous catalysts work
They work by combining with the reactants to form an intermediate species which then reacts to form the products and reform the catalyst.
Explain how the number of active sites can be increased for a given mass of catalyst.
Number of active sites increases if surface area is increased. This can be done by turning the catalyst into a powder or spreading it over a ceramic honeycomb type of support.
The efficiency of a heterogeneous catalyst often decreases during use. Explain, using a specific example, why this happens.
Active sites blocked by another species or poison. Sulphur compounds in the Haber process or lead in a catalytic converter.
Give one reason why impurities in the reactants can cause problems in processes that use heterogeneous catalysts.
Impurities block the active sites of the catalyst. Impurities adsorbs onto catalyst AND reduces surface area.
Discuss why different catalysts have different activities.
If adsorption is too weak then reactants arent brought together e.g. silver. If adsorption is too strong, products not desorbed e.g. tungsten
Explain how heterogeneous catalysts work, give one example of a reaction catalysed in this way.
Reactants first adsorb onto the catalyst's surface, where they can react. Afterward, the product molecules desorb from the surface. An example is the Haber-Bosch process for ammonia synthesis, where nitrogen and hydrogen gases react on the surface of a solid iron catalyst.
Suggest how poisoning of a catalyst, used in an industrial process, can be minimised.
Purify reactants. Remove impurities
Langmuir Model Assumptions
The surface of the adsorbent is uniform and and energetically equivalent everywhere.
Adsorption cannot proceed beyond monolayer coverage.
The surface as a fixed number of identical adsorption sites.
Each adsorption site can only accommodate one molecule.
The ability of a molecule to adsorb at given site is independent of the occupation of neighboring sites.

Adsorption on Solid Surface
Type 1
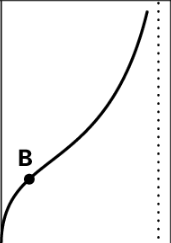
Adsorption on Solid Surface
Type 2
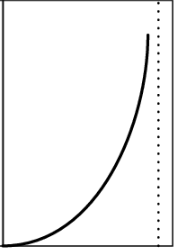
Adsorption on Solid Surface
Type 3

Adsorption on Solid Surface
Type 4
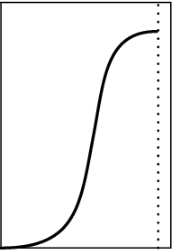
Adsorption on Solid Surface
Type 5
BET Model Assumptions
Gas can adsorb onto a solid surface in multiple layers.
Heat of Adsorption for the 1st layer is constant and different from that of subsequent layers.
Heat of adsorption of additional layers (>1) is equal to latent heat of vaporization (condensation).
Based on the Rate of Adsorption = Rate of Desorption for each layer of adsorption.
Freundlich Model
Energetically heterogeneous adsorbent surface which allows for multiplayer adsorption.
Assumes that adsorption energy exponentially decreases as the surface becomes more saturated.
Not applicable at high pressures .
Limitations of the Freundlich Model
Purely empirical with no theoretical basis
Only valid at certain temperatures
Constants k and n vary with temperature
The isotherm fails when the concentration of the adsorbate is very high.