3.1.1 - Nomenclature
1/57
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
What are organic compounds
Covalent compounds of the element carbon (C)
What are hydrocarbons
Compounds consisting of hydrogen and carbon ONLY
What is it meant by saturated
Contains Single C-C bonds only
What is it meant by unsaturated
Contains a C=C double bond
What is a homologous series
Compounds which have the same functional group
What are the characteristics of a homologous series
members of homologous series:
have same General formula
almost have same chemical properties due to same functional group
Molecular formula differ by CH₂
Show gradual change in physical properties (eg. Boiling Point)
What is a functional group
The reactive part of an organic molecule
What is the functional group for alkanes
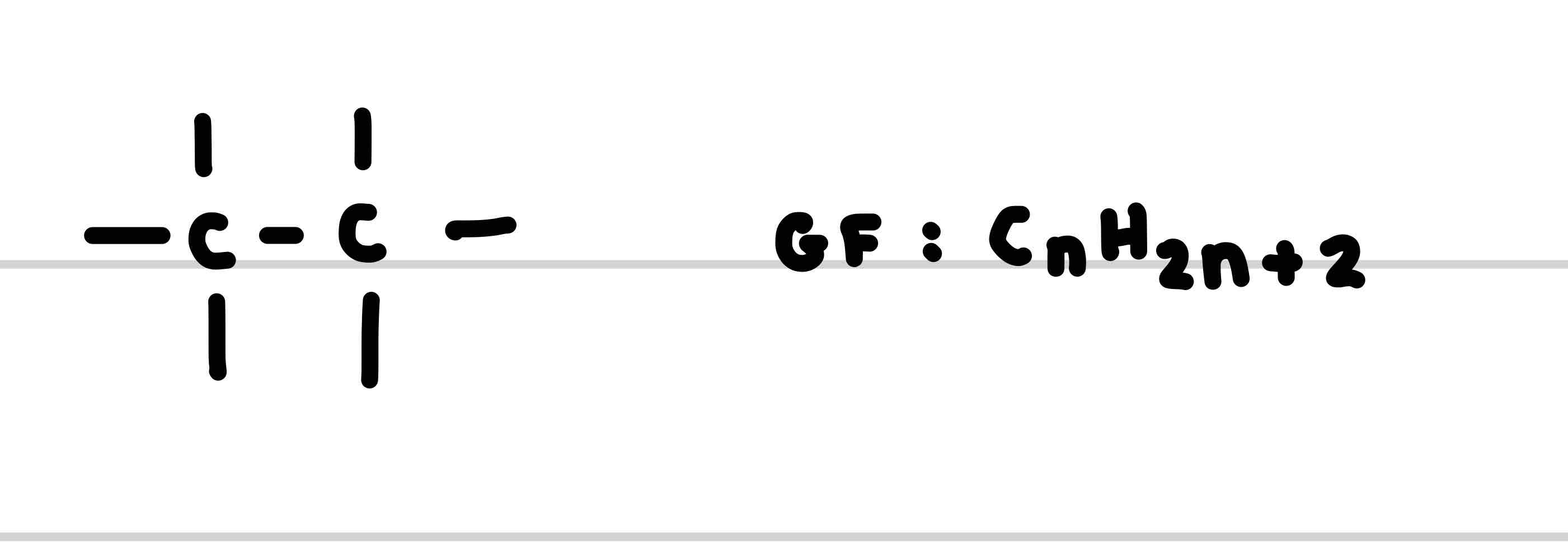
What is the suffix of the functional group alkanes
-ane
Give an example of an alkane
Propane
What is the functional group for Alkenes
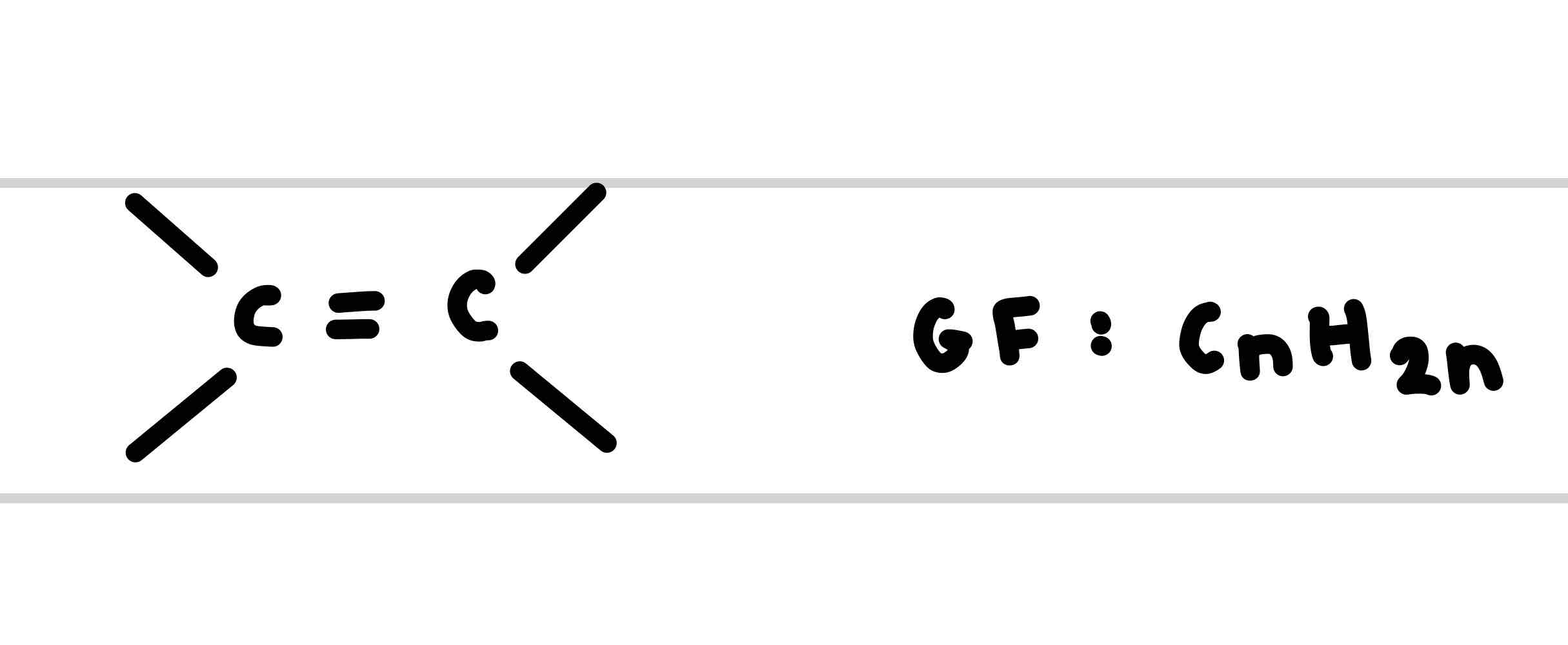
What is the suffix of the functional group alkene
-ene
If there is more than one double bond what is the suffix of the alkene
-diene (2)
-triene (3)
The stem of the carbon chain ends with an -a
eg. Penta-1,3-diene
How is an alkene named if an alcohol or Carboxylic acid functional group is present aswell
The suffix -en can go in front of the alcohol/Carboxylic acid suffixes
eg. but-2-enoic acid
Why does this happen
The alcohol and Carboxylic acid functional group have higher priority than the alkene group so take precedence (more importance) with numbering
Give an example of an alkene
But-2-ene
What is the functional group for alcohols
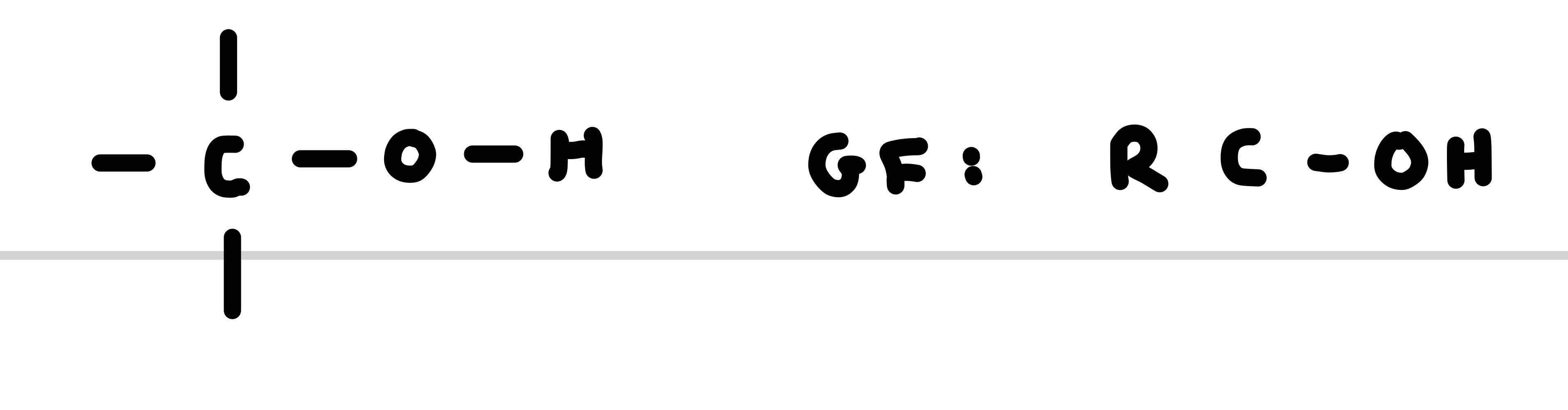
What is the suffix for alcohols
-ol
How would you name the molecule which has 2 -OH groups
Suffixes:
-diol
-triol
If there are more than one -OH add ‘e’ on stem name
eg: ethane-1,2-diol
How would you name the molecule with an alcohol along with another functional group
The priority group gets the suffix ending and the -OH named using prefix hydroxy-
eg: 2-hydroxypropanoic acid
Where does the priority groups stem from
Aldehydes
∴ Carboxylic acids are a priority group as aldehydes which are oxidised become Carboxylic acids
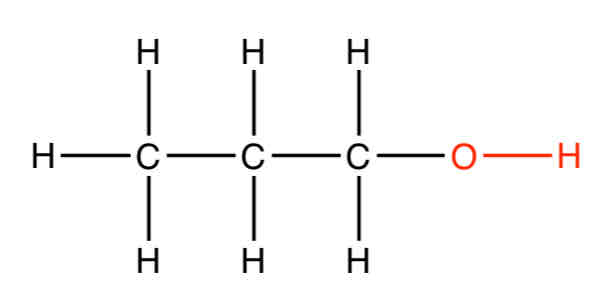
Give an example of an alcohol
Propa-1-ol
What is the functional group for aldehydes
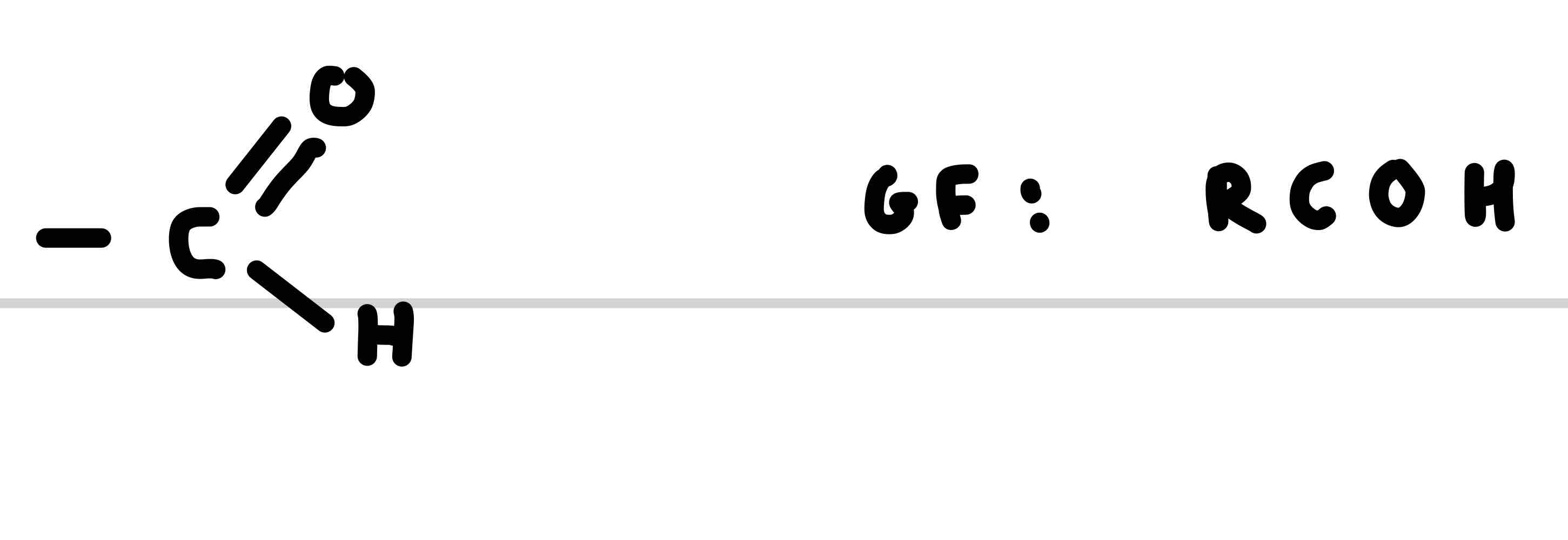
What is the suffix for aldehydes
-al
How would you name the molecule if there is more than one aldehyde functional group
Suffix: -dial
Add ‘e’ to the stem
eg: pentanedial
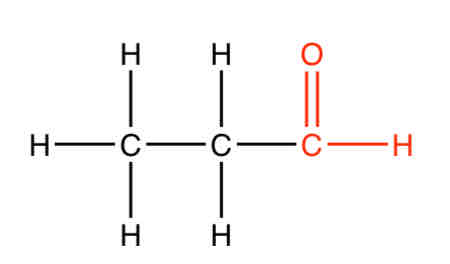
What is an example of an aldehyde
Propanal
What is the functional group of a ketone
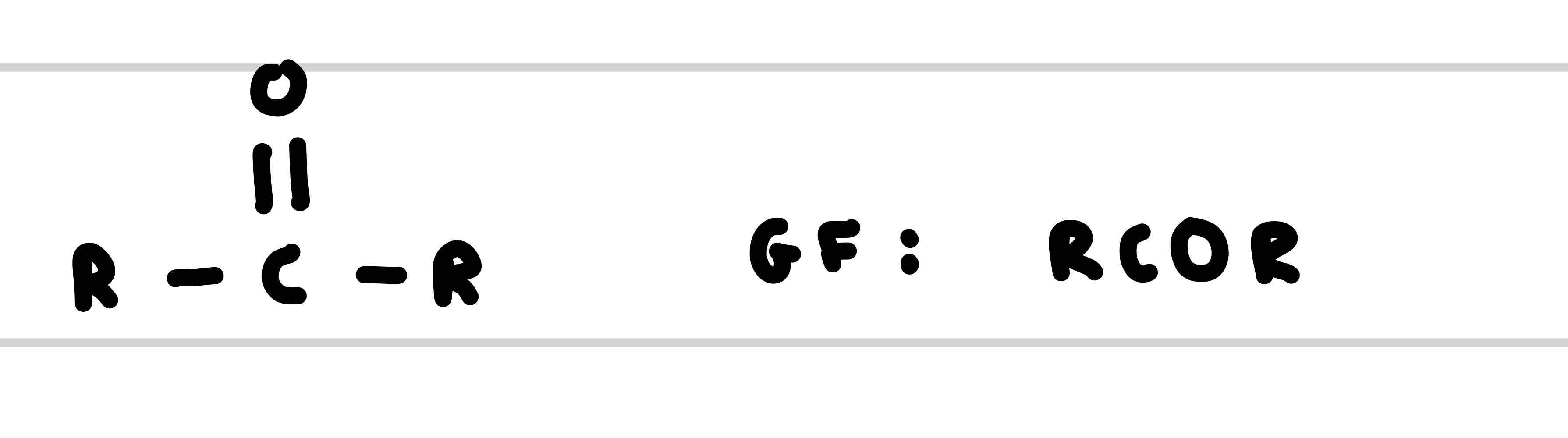
What is the suffix of a ketone
-one
How would you name the molecule if there is more than one aldehyde functional group
Suffix: -Dione
Add ‘e’ to the stem
eg: pentane-2,4-dione
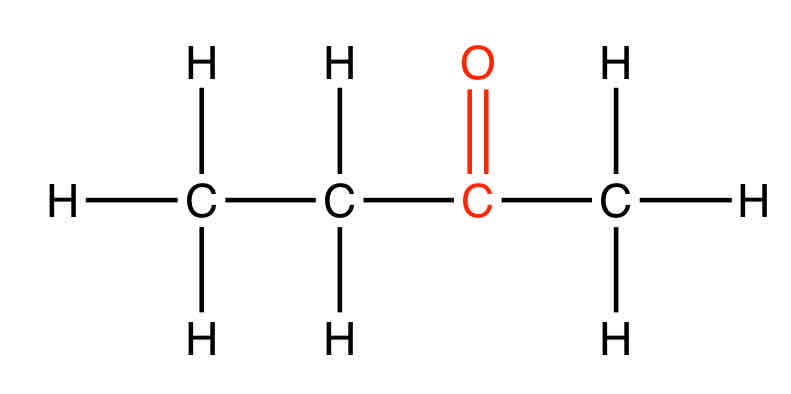
Give an example of a ketone
Butanone
What is the functional group of carboxylic acid
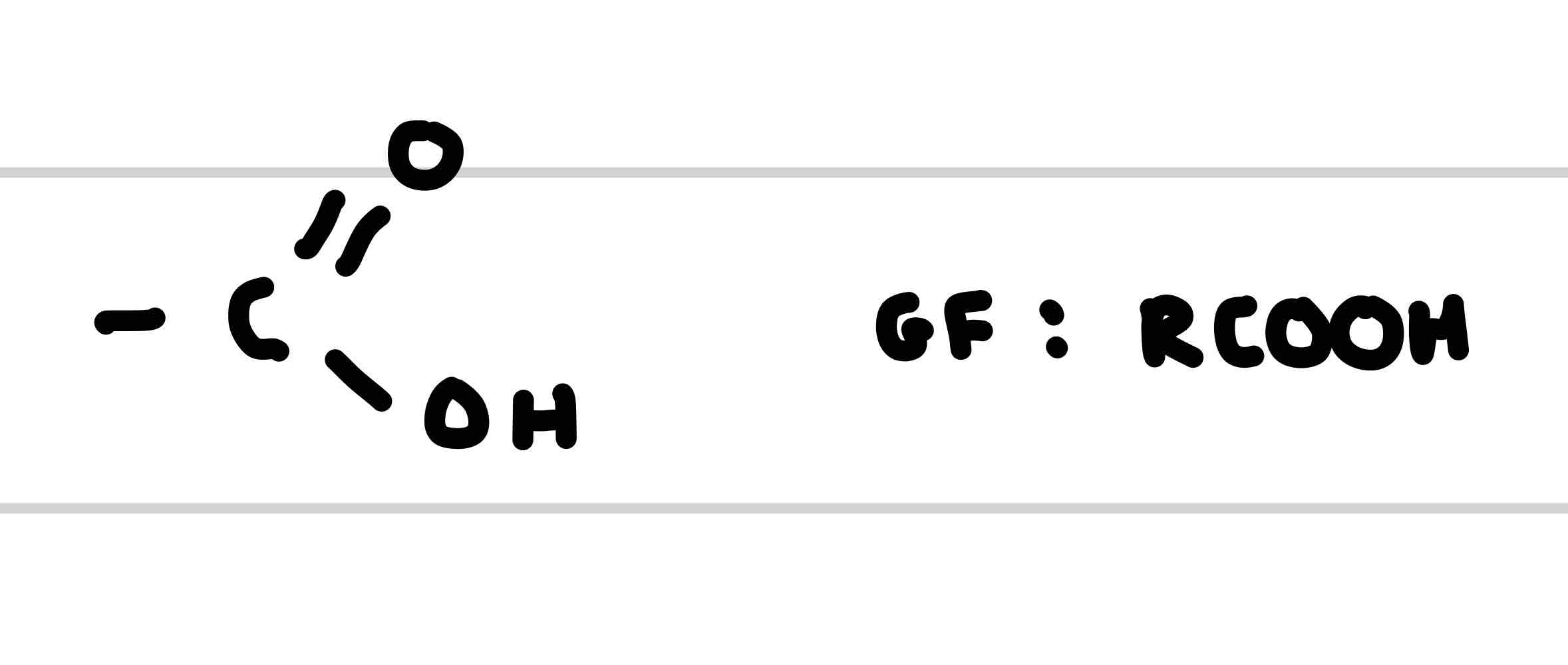
What is the suffix of a Carboxylic acid
-oic acid
How would you name the molecule if there is more than one carboxylic acid functional group
Suffix: -dioic acid
Add ‘e’ to the stem
eg: Ethanedioic acid
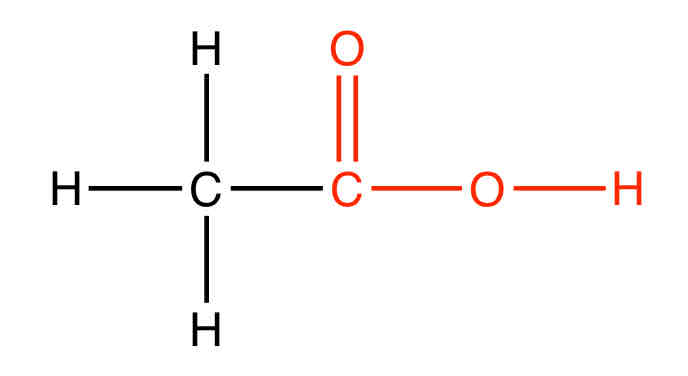
What is an example of a Carboxylic acid
Ethanoic acid
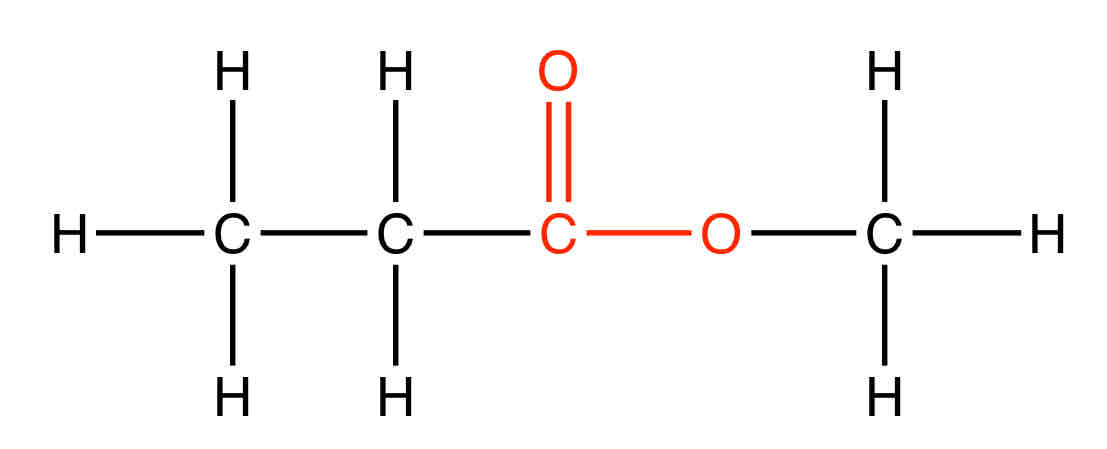
What is the functional group of an ester

What is the suffix of an ester
-oate
What is an example of an ester
Methyl propanoate
What is the functional group of a Halogenoalkane
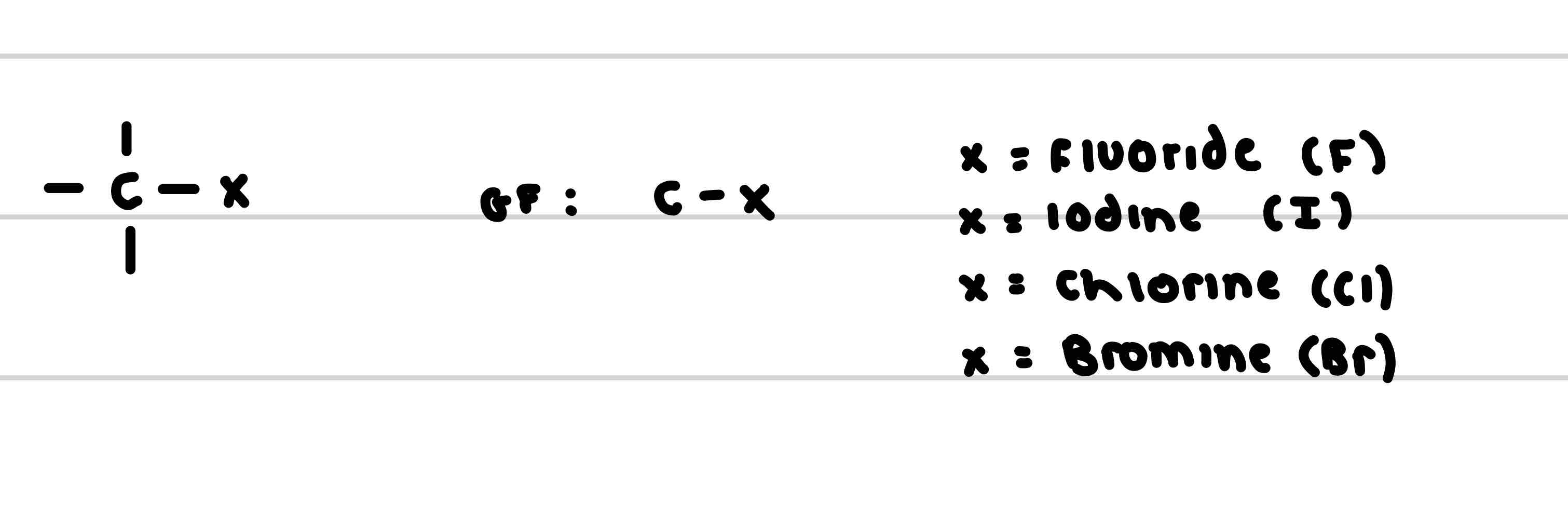
What are the prefixes of a Halogenoalkane
-fluoro
-chloro
-bromo
-iodo
How are Halogenoalkanes named with multiple functional groups
It’s listed in alphabetical order (ignores the di, tri)
eg. 5,5-dibromo-4-iodo-3-methylpent-1-ene
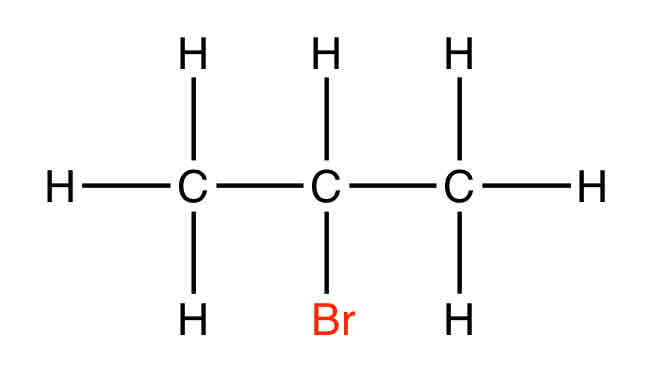
What is an example of a Halogenoalkane
2-bromopropane
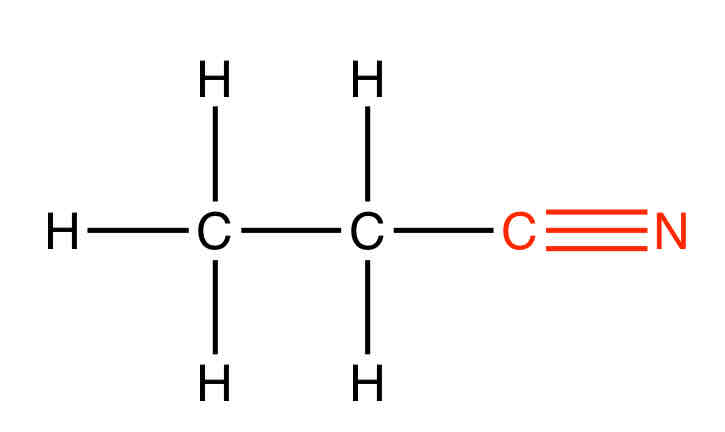
What is the functional group for a nitrile

What is the suffix for a nitrile
-nitrile
Give an example of a nitrile
Propanenitrile
What are the 6 ways organic compounds can be represented by
empirical formula
Molecular formula
General formula
Structural formula
Displayed formula
Skeletal formula
What is a empirical formula
The simplest whole no. ratio of atoms of each elements in a compound
What is a molecular formula
The true numbers of atoms of each element in a compound
What is a General formula
the algebraic formula for homologous series
Give an example of a General formula
Eg. Alkenes = CₙH₂ₙ₊₂
What is a structural formula
Shows the structural arrangement of atoms within a molecule
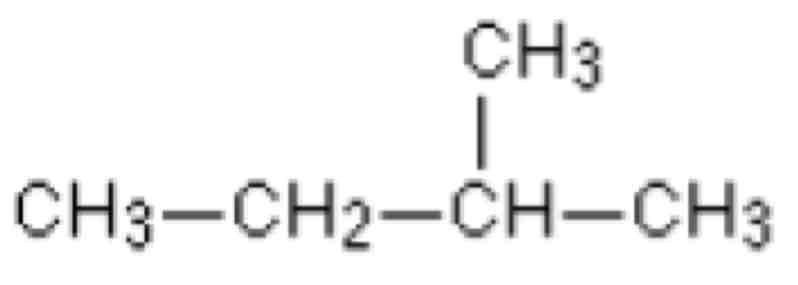
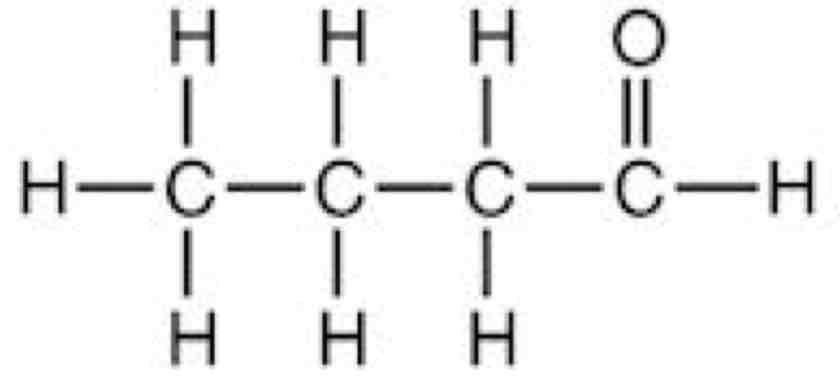
What is the displayed formula
Shows every atom and every covalent bond in an organic compound
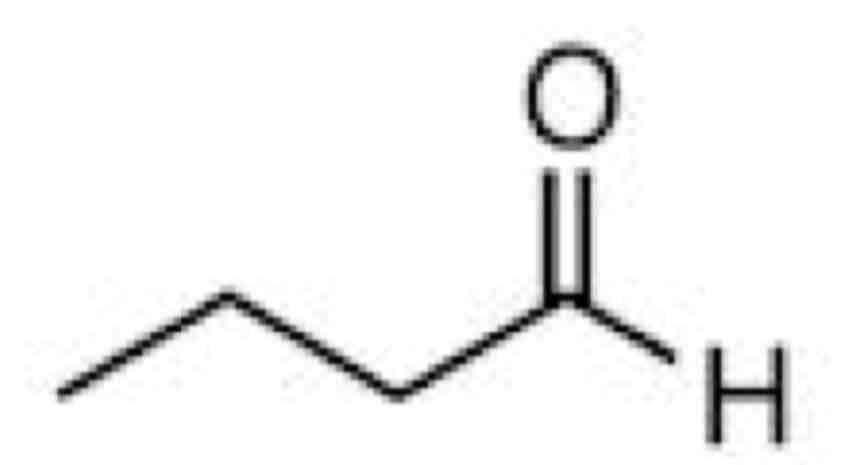
What is a skeletal formula
Shows only the bonds in the compound and any non carbon atom
With the assumption Hydrogen is bonded to the carbons unless stated otherwise
What is the shape around carbon atoms in saturated hydrocarbons
Tetrahedral
What is the bond angle around carbon atoms in saturated hydrocarbons
109.5°
What are the general rules for naming carbon chains
use commas between numbers
Use dashes between numbers and words
Put numbers in ascending order
What is the general rule for naming functional groups
If the suffix of the functional group begins with a vowel remove the -e from the stem alkane name
eg: propane-1-ol x
propan-1-ol ✓
How do you name the molecule when there a 2+ of the same functional group
You name them:
di- (2)
tri- (3)
tetra- (4)
penta- (5)
hexa- (6)
How do you name a molecule with 2 different functional groups
The functional groups are listed in alphabetical order regardless of where on the carbon chain it’s located
eg. 3-bromo-1-fluoropentane