W3- Pathology of inflammatory responses
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
what is produced in an acute inflammatory response?
inflammatory exudate (fluid rich in protein and cells)
what causes oedema in the acute inflammatory response?
microvascular changes cause increased blood vessel permeability
what is oedema?
excess accumulation of fluid in extravascular space and body cavities
due to increased vascular permeability, increased hydrostatic pressure or decreased colloid oncotic pressure
what stimulates the process of destruction of the pathogen and other cells in the local environment?
-if fluid in interstitial spaces contains inflammatory cells (eg neutrophils)
-toxic metabolites released to cause destruction
what is pus?
purulent exudate made of damaged tissue and dead microbes, granulocytes, and macrophages
why is it useful that exudates contain fibrinogen?
fibrinogen converted to fibrin - forms a mesh for healing and repair in coagulation cascade
what are the 5 clinical symptoms of acute inflammation?
-redness
-heat
-swelling
-pain
-loss of function
what pathway of the complement cascade does the acute inflammatory response involve?
alternative pathway (based on pathogen surface binding)
what are the 4 processes which occur after binding of a pathogen to complement C3 and activating complement cascade?
-release of histamine and chemoattractants
-altered permeability of local vasculature that allows fluid exudation (oedema)
-altered adhesiveness of endothelium allows cellular migration (extravasation by diapedesis)
-phagocyte recognition of C3b-opsonised pathogen allows it to be engulfed and destroyed
how is the complement cascade activated?
pathogen binds complement C3
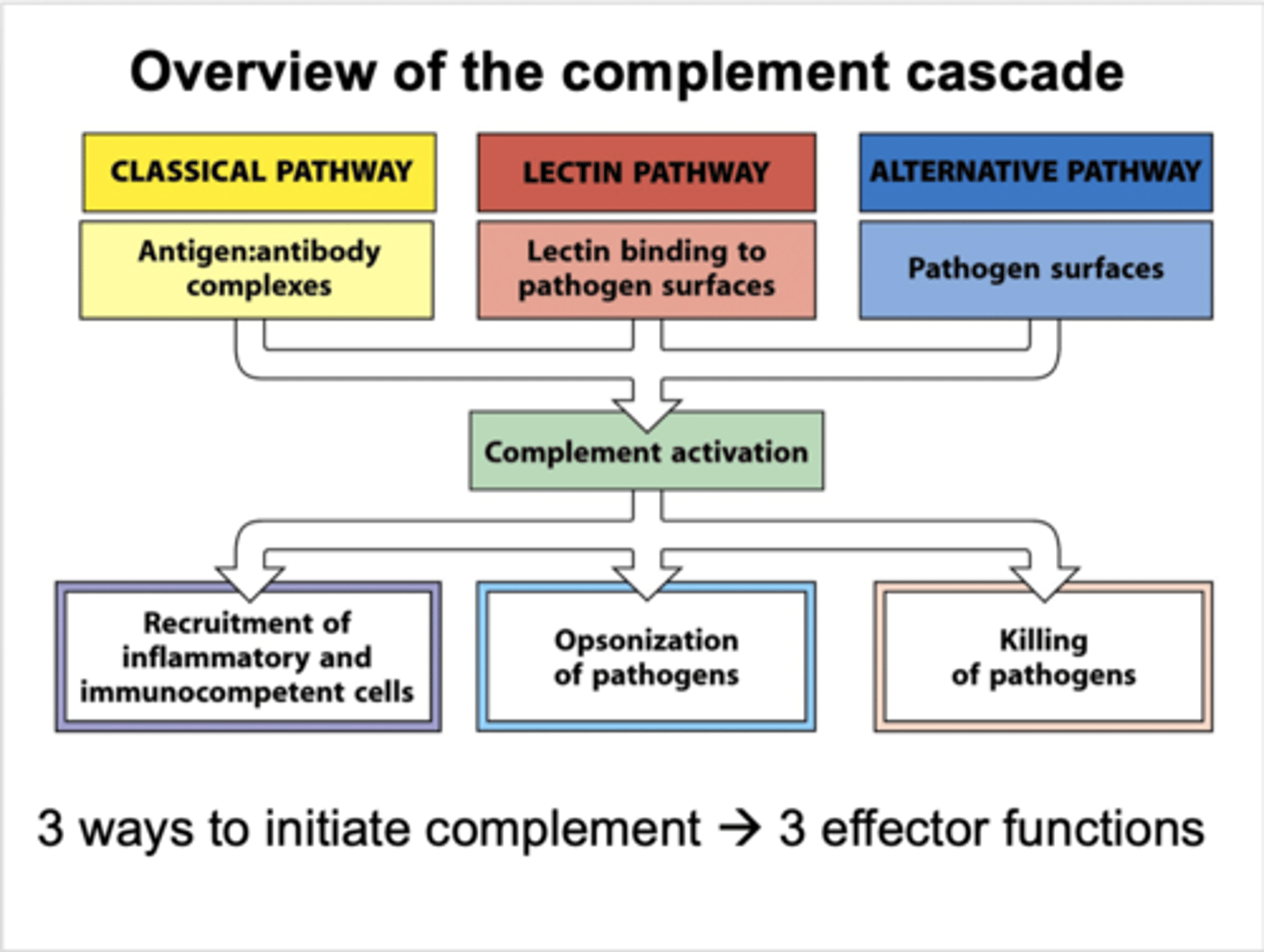
what are the events of the alternative pathway?
-pathogen attaches to immature C3
-causes thioester hydrolysis to activate C3 convertase
-activates common pathway
what occurs in the common pathway of the complement cascade?
C3 convertase --> C3 --> C3a and C3b
C3b --> C5 convertase --> C5
C5 --> C5a and C5b
what is the function of C3b from the complement cascade?
opsonisation- coats bacteria for recognition and engulfing by phagocytic cells
what is the function of C3a and C5a from the complement cascade?
activation of mast cells to release vascular permeability factors and chemoattractants
(allows inflammatory exudate cells to respond to signal at site of infection)
what is the function of C5b-C9 from the complement cascade?
activates membrane attack complex where pathogens are destroyed in cell lysis
what are the 3 functions of cellular and plasma mediators of acute inflammation?
-amplification
-elimination
-regulation
what are some examples of cellular mediators of inflammation?
-vasoactive amines
-cytokines and growth factors
-arachidonic acid derivatives (thromboxane A2, prostaglandins)
-platelet activating factor
-lysosomal enzymes
-oxygen radicals
-nitric oxide
what are some examples of plasma-derived mediators of acute inflammation?
-kinin system
-coagulation and fibrinolytic system
-complement cascade
how is platelet activating factor produced? (cellular mediator of acute inflammation)
phospholipid molecule derived from cell membrane of basophils, macrophages, mast cells or endothelial cells
what is the potent pathological effect of platelet activating factor?
contributes to:
-inflammation
-endotoxin shock
-allergic reactions (through vasoconstriction and bronchoconstriction)
-leukocyte adhesion
-platelet aggregation
what is different about the systemic effects of low conc platelet activating factor v high conc platelet activating factor?
low conc= vasodilation
high conc= vasoconstriction
what are the 3 main functions of platelet activating factor as a cellular mediator of acute inflammation?
-enhances microbicidal activity in neutrophils through oxidative burst
-enhances phagocytosis
-activates arachidonic acid pathway to produce leukotrienes, prostaglandins and thromboxane
what are the 3 major pathways for regulating acute inflammation?
1. complement cascade
2. platelet activating factor
3. arachidonic acid metabolic pathway
what 3 substances activate the arachidonic acid pathway?
-C3a
-C5a
-platelet activating factor
what are the two pathways involved in the arachidonic acid pathway?
what do they produce?
-cyclo-oxygenase pathway = prostaglandin and thromboxane A2 production
lipo-oxygenase pathway = leukotriene production
what is the function of thromboxane A2 produced in the arachidonic acid pathway?
vasoconstriction and platelet aggregation
what is the function of prostacyclin produced in the arachidonic acid pathway?
platelet disaggregation
what is the function of prostaglandins produced in the arachidonic acid pathway?
vasodilation
what is the function of leukotrienes produced in the arachidonic acid pathway?
-chemotaxis and neutrophil adhesion
-increased vascular permeability
-vasoconstriction
what are the 3 processes/substances that lead to systemic effects from localised acute inflammation?
-acute phase reactions
-acute phase proteins
-exogenous/endogenous pyrogens
what are acute phase proteins?
proteins whose plasma concentrations increase (positive acute-phase proteins) or decrease (negative acute-phase proteins) in response to inflammation
why are acute phase proteins produced in inflammation?
due to altered liver metabolism
what are some examples of acute phase reactions which are caused by production of acute phase proteins in the liver in response to local inflammation?
-increased bone marrow leukocyte production (more neutrophils from bone marrow and other reservoirs required)
-fever
-rigors
-tachycardia
-drop in BP
-loss of appetite
-vomiting
-skeletal weakness
-aching
what is the substance that causes fever?
pyrogens
how do pyrogens control fever?
reset temperature control system operated by hypothalamus
what is an example of an exogenous pyrogen?
lipopolysaccharide from Gr- bacteria (endotoxin)
what are examples of endogenous pyrogens?
cytokines (IL-1, IL-6, TNF) - involved in acute inflammation
why does fever cause general malaise?
due to interaction of hypothalamic-pituitary-adrenal axis
(causes widespread systemic effects)
what are the properties of acute v chronic inflammation?
acute
-short-lived (mins-days)
-neutrophil-rich
chronic
-long-lived (months-years)
-lymphocyte, plasma cells and macrophage-rich
what are the 5 stages of resolution of inflammation? (acute inflammation)
1. injury/infection
2. acute inflammatory response (histamine, 5-HT, complement)
3. lipoxins and resolvins
4. resolution
5. return to normal tissue physiology
what are the 5 stages involved in the failure to resolve inflammation? (chronic inflammation)
1. injury/infection
2. acute inflammatory response (histamine, 5-HT, complement)
3. lipoxins and resolvins
4. 'failed resolution'- ongoing acute inflammation
5. maintains persistent state of acute inflammation = chronic inflammation
what are 3 characteristics of chronic inflammation?
-abscess formation
-excess scarring
-autoimmunity
why is resolution of infection an active (not passive) process?
requires specific molecular and cellular pathways to be activated
what are 3 substances involved in resolution of acute inflammation?
lipoxins, resolvins and protectins
why is it important to understand the active processes which trigger resolution?
provides candidates for drug targets
(can modify inflammatory process and enhance resolution when it is not working in chronic inflammation)
what are the 4 types of chronic inflammation?
-non-specific
-chronic suppurative
-granulomatous
-autoimmune
what are the properties of non-specific chronic inflammation?
-persistent viral infection (eg Hep B) continues to stimulate the local immune response
-associated with persistent activation of lymphocytes
what are the properties of chronic suppurative inflammation?
-abscess-continuing stimulus to neutrophil production and recruitment
-often walled off by fibrous tissue and poorly vascularised, so difficult to treat with antibiotics
-may require surgical clearance
what are the properties of granulomatous chronic inflammation?
-usually a response to agents which are difficult to destroy using lysosomal enzymes or lymphocyte-mediated immune responses
-epithelioid or multinucleated giant cells form by fusion
(eg TB, parasitic infection)
what are the properties of autoimmune chronic inflammation?
-involves T, B and plasma cells
-plasma cells may be a prominent feature in inflammatory infiltrate
-can result in the development of tertiary lymphoid structures
what are tertiary lymphoid structures? what do they indicate?
-rudimentary organelles that function similarly to lymph nodes and lymphoid tissues
-found in autoimmune chronic inflammation
-development is a poor prognostic indicator of autoimmune progression
what are some examples of diseases which are underpinned by chronic inflammation?
-atherosclerosis
-arthritis
-chronic neurodegenerative diseases (eg Alzheimer's)
-dementia
-depression
what are the 4 stages of acute inflammation in lobular pneumoniae?
1. congestion
2. consolidation
3. grey hepatisation
4. resolution
what occurs in the congestion phase (phase 1) of acute inflammation in lobular pneumoniae?
-bacteria cause outpouring of protein-rich (fibrin) fluid into alveolus
-first 24 hours after infection
presence of bacteria and neutrophils, but not blood cells
what occurs in the consolidation phase (phase 2) of acute inflammation in lobular pneumoniae?
-occurs 2-3 days after insult and lasts 2-4 days
-neutrophils and rbc enter alveolus
-fluid and cells spread to adjacent alveoli, leading to solidification
-marked with cellular exudate (neutrophils with ingested bacteria, extravasated rbc, epithelial cells, fibrin deposits)
-fibrin deposition replaces the fluid from the congestion phase
why is the consolidation phase of acute inflammation in lobular pneumoniae known as 'red hepatisation'?
texture appears to be like liver
what are the properties of the affected lung in lobular pneumoniae in the consolidation phase?
dry, granular, airless
what occurs in the grey hepatisation stage (phase 3) of the acute inflammatory response in lobular pneumoniae?
-lasts up to 8 days
-macrophages recruited to ingest dead neutrophils and begin digestion of fibrin mesh
what are the properties of the affected lung in lobular pneumoniae in the grey hepatisation stage?
-grey due to fibrin exudate (fibrin being digested by macrophages)
-disintegration of rbc leads to deposits of haemosiderin
-texture= cooked liver
what occurs in the resolution phase (phase 4) of the acute inflammatory response in lobular pneumoniae?
-restoration of normal tissue when architecture is in tact
-begins centrally in lobe and spreads peripherally
-liquifies fibrous content from earlier phases to recover aeration in the alveoli
(occurs after 8 days)
what occurs when resolution is not possible in the acute inflammatory reaction?
repair- doesn't restore normal architecture, but allows continued life
what is repair after the acute inflammatory response associated with?
organisation- scar formation due to loss of structural integrity
what is an abscess?
accumulation of neutrophils walled off by fibrin and often surrounded by chronic inflammatory cells (macrophages and fibroblasts)
why does an abscess suggest a chronic condition of persistent acute inflammation?
acute inflammation= accumulation of neutrophils
chronic condition= fibrin and chronic inflammatory cells (macrophages and fibroblasts) preventing resolution of acute inflammation
what is suppurative inflammation?
condition where purulent exudate is accompanied by significant liquefactive necrosis
(large amounts of pus due to dense accumulation of neutrophils in the abscess)
what is suppurative inflammation difficult to treat with antibiotics?
-poorly vascularised
-fibrin wall surrounding neutrophils prevents penetration
(may require surgical excision)
what is granulomatous inflammation?
histological pattern of chronic inflammation which occurs following cell insult
what are the various causes of granulomas?
fungi
bacteria
mycobacteria
chemical
autoimmune
cancer
metazoa
protozoa
what stain do we use to observe nuclear and cytoplasmic morphology of granulomas?
H+E stain
why are macrophages found within granulomas?
granulomas are sites of chronic inflammation
what happens to macrophages in granulomas?
take on unusual shapes or characteristics
what are the two most common macrophage shapes/characteristics in granulomas?
-epithelioid cell- macrophages start to resemble (but do not transform into) cuboidal epithelial cells
-macrophages acquire multiple nuclei
what does the presence of epithelioid cells in the granuloma indicate?
chronic immune stimulation of macrophages in granuloma
what are the two reasons that macrophages acquire multiple nuclei in the granuloma?
-failure of cell division with nuclear division still occurring
-fusion of multiple macrophages within granuloma to give rise to large cytoplasmic mass with multiple nuclei contained within
what are the 4 types of multi-nuclear macrophages that are formed in the granuloma?
-foreign body cell- random distribution of nuclei
-langhan giant cell- horse-shoe arrangement of nuclei (eg TB)
-Warthin-Finkeldey cell- contain inconspicuous nuclei
-touton giant cell- nuclei arranged in centre of cell and surrounded by lipid bodies
what are the properties of a non-caseating (sarcoid) granuloma?
-non-necrotising (absence of dead cells in the core)
-macrophage-rich core surrounded by lymphocytes (CD4+, CD8+)
-network of fibroblasts surrounded granuloma
(raised structure if appears on skin)
what are the properties of caseating granuloma?
-macrophage-rich core surrounded by lymphocytes in a cuff
-infiltration of neutrophils/polymorphic cells resulting in a caseating centre (dead cells in centre)
-acute inflammation continues in centre (neutrophil-rich) at same time as chronic inflammation
how is formalin fixed paraffin embedded tissue used when looking at granulomas?
useful to understand the dynamics of the inflammatory process in animal models
(how granulomas are formed and maintained)
how are formalin fixed paraffin embedded tissue slides produced?
-chemicals to allow hydration and dehydration of tissue
-machine allowing to embed fixed material into paraffin
-machine allowing to cut thin sections of the block
-animal tissue can be embedded into paraffin blocks to cut the tissue so it can be viewed microscopically
why is using formalin fixed paraffin embedded tissue microscopy useful?
can look at nuclear and cytoplasmic morphology of granulomas over time
what other use do formalin fixed paraffin embedded sections have> (not staining and microscopy)
formalin fixed paraffin embedded sections can yield molecular information through genome techniques
(static, but can capture more info than by H+E staining or immunofluorescent staining)
how are formalin fixed paraffin embedded sections used in application of genome techniques to study molecular pathology?
-FFPE blocks are cut to produce FFPE slides with sections of tissue
-DNA is extracted from tissues
-next generation sequencing technologies can be used to understand genetic makeup of cells in tissues
how is genetic technology applied to the genetic sequences from the formalin fixed paraffin embedded tissues to understand the transcriptome (what genes are being expressed as proteins by the tissue)?
-based on RNA analysis (RNA sequencing of FFPE sections)
-minute amounts of RNA are extracted from FFPE sections
-RNA sequencing analysis is conducted
-this builds up a gene expression profile of different cells within tissue
what occurs in immunostaining technology when looking and granulomas?
-used to expand on info from H+E staining
-identifying cell types based on molecules rather than morphology
-take antibody molecules which recognise different characteristics of cell types and label them with specific dye under UV light
-allows for greater understanding of granuloma (types of cell, position of cells relative to other cells, number of cells)
what is the process involved in immunostaining granulomas?
-monoclonal antibodies are stained an placed on a section
-visualised under a camera equipped with UV light source which captures images digitally
what is involved in intravital fluorescence multiphoton microscopy for observing granulomas?
intravital = in live animals (visualising cell dynamics)
multiphoton microscopy = allows good depth penetration into tissues at high depth resolution to be able to see individual cells and how they behave
why is intravital fluorescence multiphoton microscopy useful for identifying granulomas?
-allows dynamic impressions of inflammatory response associated with granuloma
-specific cells in granuloma can be labelled individually in distinct ways (capture differences in shape, size, motility of inflammatory cells)
-can view different types of cells associating with granuloma moving in and out over time
what are the properties of intravital and non-invasive probes which are being developed for looking at granulomas?
small microscopic objectives placed at end of a small catheter into live animals for representation of tissue structures and dynamics during the course of infection
(may be able to be done in humans in the future)
what is an example of non-invasive imaging that can be used to look at granulomas?
functional MRI- imaging technique to look at details of immune response in humans in real time
how does cytometry work to look at cells involved in granulomas?
identifies individual cell populations based on cell surface characteristics
(flow cytometry= 10-20 molecules)
(mass cytometry= 100 molecules)
what is the process of cytometry for looking at cells involved in granulomas?
-small pieces of tissue are disaggregated and passed through cytometer
-allows interrogation of surface molecule expression of each cell in population
why is cytometry not always used for looking at cells involved in granulomas even though it is the best method in cell phenotyping?
causes loss of spatial info about cells and structures surrounding the m
(disaggregation means you can no longer understand where cells are in relation to each other/which part of the tissue they came from)
what is digital space profiling when looking at granulomas?
-combines spatial analysis with genomics platforms
-uses highly multiplexed antibody and RNA probes
what is the process of digital spatial profiling?
-stain slide tissue section with RNA probe
-image slide and select regions of interest
-UV cleave RNA off antibodies in the region of interest
-aspirate RNA with microcapillary
-dispense RNA in a plate
-repeat for each region of interest
-hybridise and count RNA
why is digital spatial profiling the best method to use for looking at granulomas?
-defines gene expression profile of individual cells while still understanding where cells are placed within a tissue section
-allows to produce more detailed maps about how cells behave in relation to one another or to pathogens at site of infection
what is in silico modelling of granulomatous inflammation?
all data acquired by various techniques integrated into mathematical models that allow us to build up architecture of a tissue
3D models allow us to try and computationally understand how granulomas behave