Chemistry Lab II Quiz- Molar Mass of a Volatile Liquid
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What is the Purpose of this lab?
1. To measure the physical properties of pressure, volume, and temperature for a gaseous substance.
2. To determine the molar mass of a volatile liquid.
Osometer
An instrument that measures changes in osmotic pressure of the solvent in which a substance, the solute, is soluble.
Mass Spectrometry
An instrumental method for identifying a gaseous ion according to it's mass and charge.
Volatile
readily vaporizable.
What are some properties of volatile liquids?
1. Low boiling point
2. low molar masses
Dumas Method
A procedure in which a liquid is vaporized into a fixed volume vessel at a measured temperature and barometric pressure.
n (vapor) =
What is the value of the R constant?
PV/RT
R= .08206
What does R stand for?
The universal gas constant
What does P stand for?
The barometric pressure in atmospheres.
What does V stand for?
The volume in liters of the vessel into which the liquid is vaporized.
What does T stand for?
The temperature in Kelvins of the vapor.
How is mVapor calculated?
From the Mass Difference from the Flask with the vapor minus the empty flask
Mflask+vapor-Mflask
How is Mcompound calculated?
(The Molar Mass of the Compound)
Mcompound=mvapor/nvapor
Molar Mass of the Compound= (mass of the vapor/moles of the vapor)
Gases and liquids with relatively large intermolecular forces and large molecular volumes ___________ behave according to the _____________.
DO NOT
Ideal Gas Law Equation
What equation can you use instead of the ideal gas law equation incase you have a gas or liquid that has large molecular volumes/ large intermolecular forces?
The Van der Waals Equation-
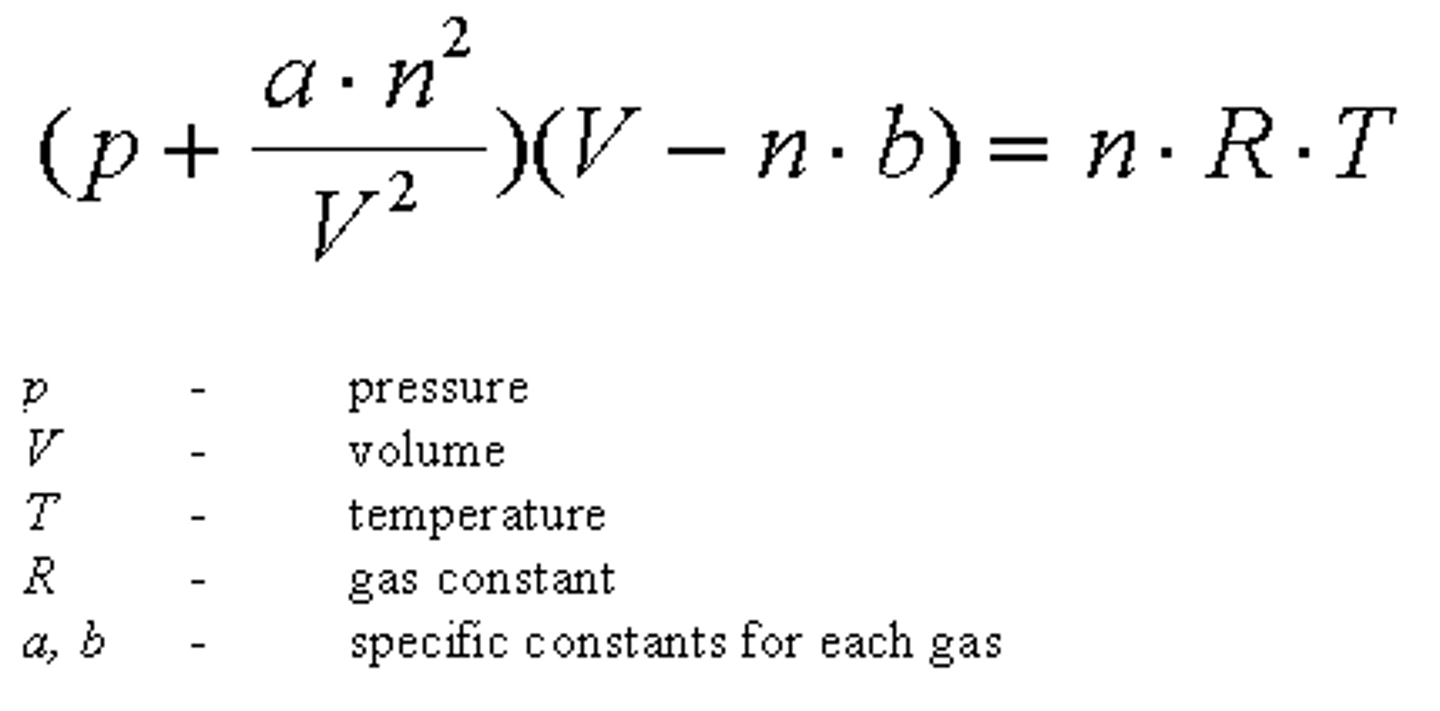
What does "a" equal in the Van der Waals equation?
"A" is representative of the intermolecular forces of the vapor
What does "b" equal in the Van der Waals equation?
"B" is representative of the volume(size) of the molecules.
How is the volume of the flask measured?
You fill the flask with water and as the flask is open to the atmosphere, you record the barometric pressure.
How do you vaporize the unknown liquid in the flask?
You boil it until it vaporizes.
How many trials are you to complete?
3
How many mL of liquid are to be obtained from your lab instructor prior to each trial?
15-20mL.
How are you to prepare the Erlenmyer flask in this experiment?
Clean the 125mL flask by drying it in a drying oven or by allowing it to air dry. DO NOT wipe it dry or heat It over a direct flame.
What are you to cover the flask's top with?
Aluminum Foil and secure It with a rubber band.
When you are obtaining the mass of the dry flask, what else are you technically measuring?
The aluminum foil and rubber band.
Explain the process you are to conduct once you have your 5mL of unknown liquid.
Transfer the 5mL of unknown liquid into the 125mL flask and place the aluminum foil and rubber band over the mouth of the flask again. With a pin, pierce little holes in the aluminum foil.
Explain the process of preparing a boiling water bath.
Half fill a 400 mL beaker with water and add one or two boiling chips to the water. Heat the beaker over a Bunsen flame or a hot plate. Secure a thermometer to measure the temperature of the water.
Boiling Chip
A piece of porous ceramic that releases air when heated. The bubbles that form prevent the water from being superheated.
Explain the process of placing the125mL flask with the sample in it into the boiling water bath in the 400mL beaker.
Lower the flask with the sample into the boiling water bath and secure it with a clamp but make sure that the clamp does not touch the beaker wall, and the flask doesn't touch the beaker wall. Adjust the water level until it is HIGH on the neck of the 125mL flask.
Explain the process of heating the sample to the temperature of boiling water.
Gently heat the water in the flask until it reaches a gentle boil. (Gentle boils are used because most unknowns are flammable.) When the liquid in the flask isn't visible or there is no more vapors escaping through the holes in the aluminum foil, continue heating for 5 minutes. Then record the temperature of the boiling water.
Explain the process of measuring the flask after you have finished the boiling water bath.
You remove the flask from the boiling water bath and let it cool to room temperature. If condensation forms on the outside of the flask just wipe it off with a towel. Then record the mass of the flask, aluminum foil and ruber band on a scale.
Explain how to measure the volume of the flask?
Fill the empty 125mL flask to the brim with water. Measure the volume of the flask by transferring the water to a 50mL or 100mL graduated cylinder.
How do you record the pressure of the vapor in the flask?
Using a barometer, record the atmospheric pressure plus one uncertain digit.