Movement of particles
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is diffusion?
The movement of particles through a liquid or gas from an area of high concentration to an area of low concentration
Name three experiments used to demonstrate diffusion:
Potassium Manganate (VII) and water
Ammonia and Hydrogen Chloride
Bromine Gas and Air
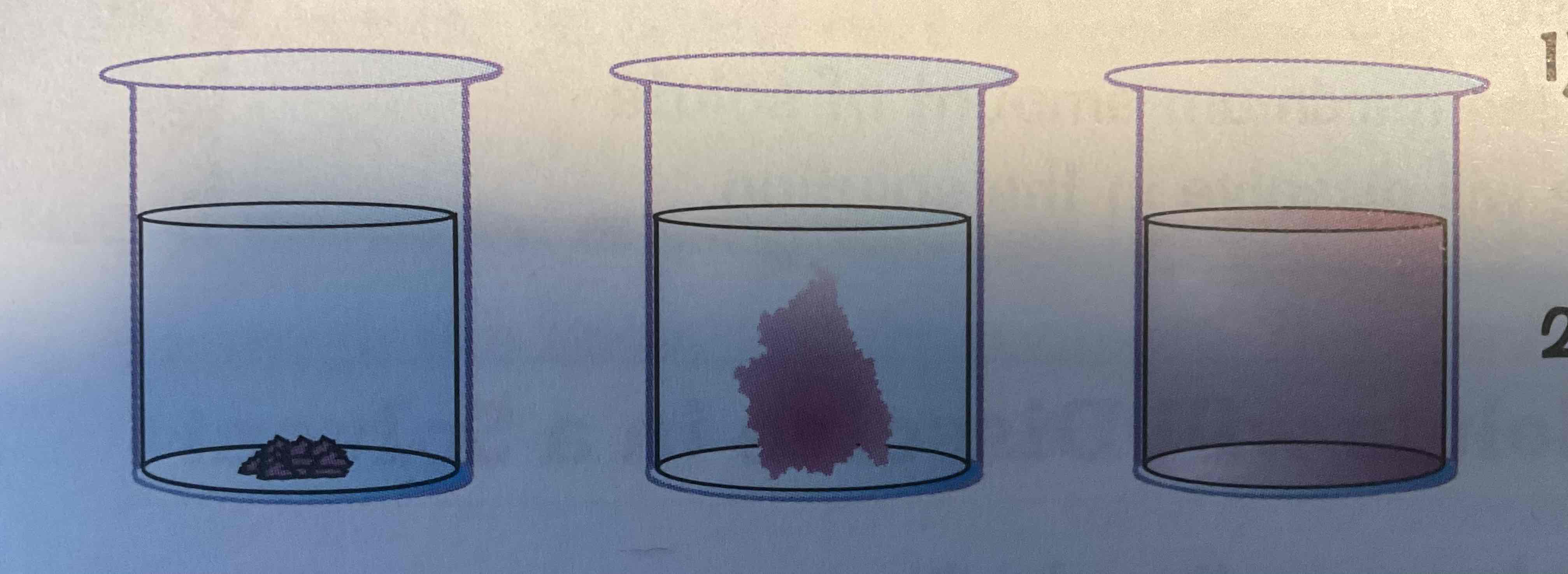
Describe the Potassium Manganate (VII) and water test to demonstrate diffusion
Potassium Manganate (VII) is bright purple
Take a beaker of water + potassium Manganate (VII) to the bottom → purple colour slowly spread out to fill beaker
Particles of potassium Manganate (VII) are diffusing out amount particles of water
Random motion of particles in liquid cause purple colour to eventually be evenly spread out
What happens when you add more water to potassium Manganate (VII) + water
Potassium Manganate (VII) particles spread out even further → less purple = dilution
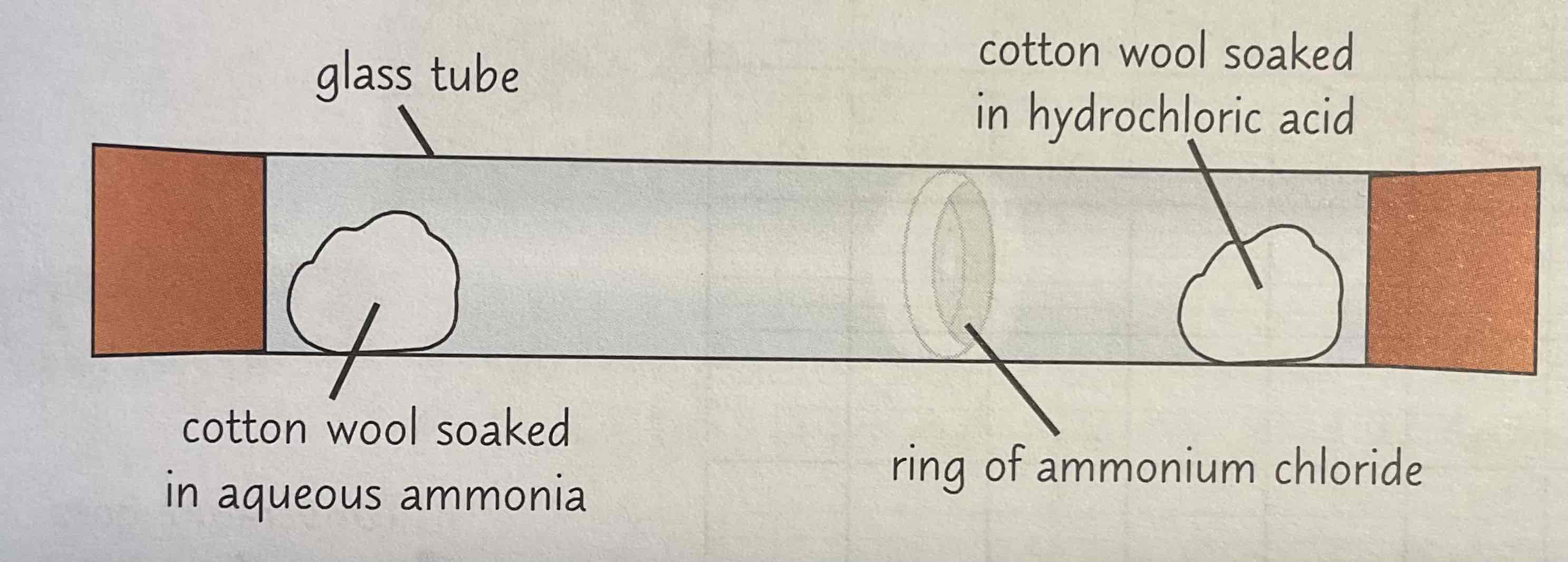
Describe the ammonium and hydrogen chloride test to show diffusion
Aqueous ammonia (NH3) gives off ammonia gas. (HCl) gives off hydrogen chloride gas.
Each gas diffuses from a different end of the tube, when they meet white ring of ammonium chloride forms
Ring dosent form in middle - forms closer to end where hydrochloric acid was
This shows that ammonia diffuses faster as it travels further in the same amount of time
Ammonia gas diffuses faster → particles of ammonia are smaller + lighter than the particles of hydrogen, so they infuse through the air more quickly
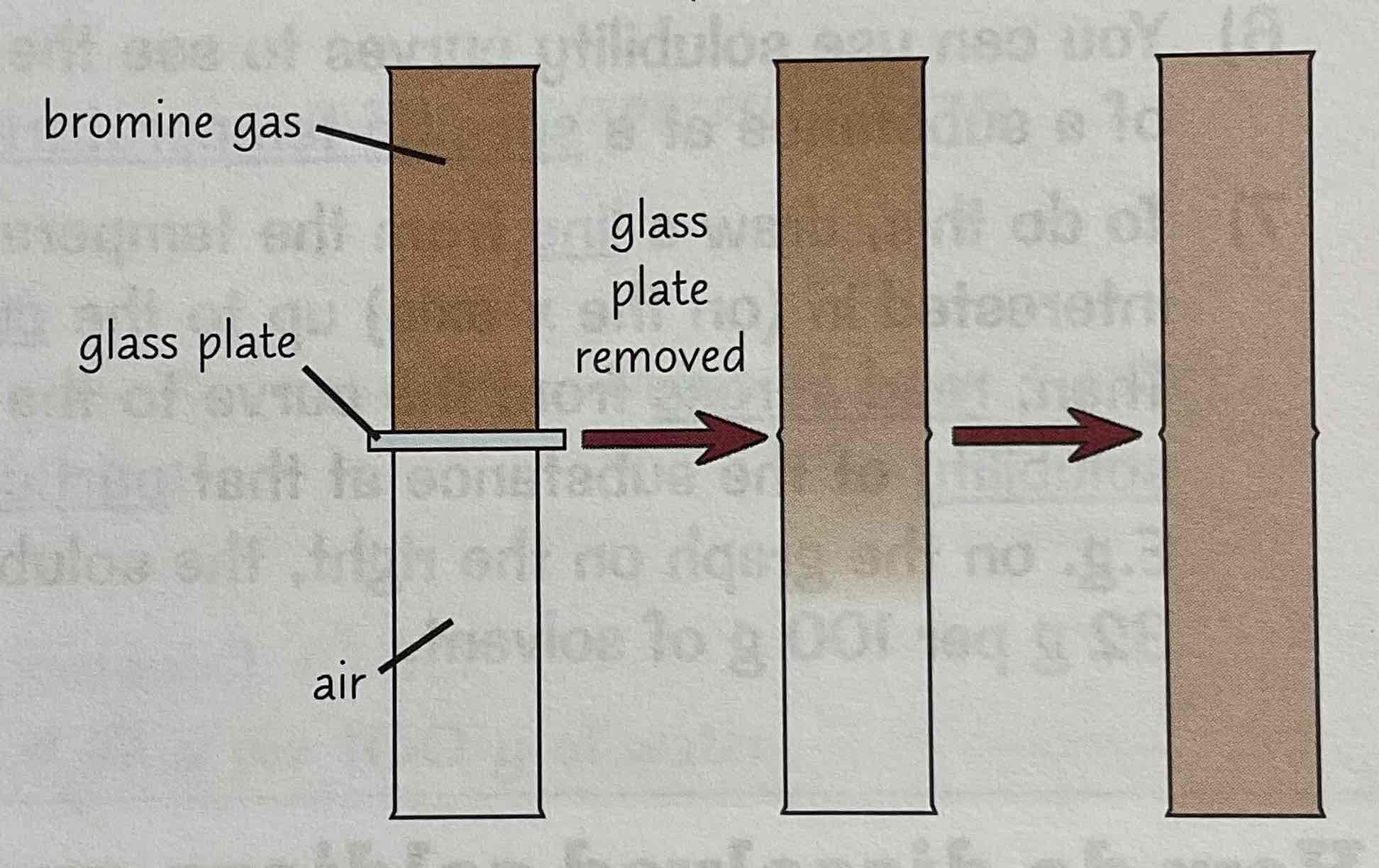
Describe the bromine gas and air test to demonstrate diffusion
Bromine gas - brown, strongly smelling - use to demonstrate diffusion in gases
Fill half a gas jar full of bromine gas - other half full of air, separate gases with glass plate
When you remove plate - see brown bromine gas slowly diffusing through air
Random motion of particles means bromine will eventually diffuse right through the air
What is a solution?
A mixture of a solvent and solute that does not separate out
How is a solution formed?
When you add a solid (the solute) to a liquid (the solvent) the bonds holding the solute molecules together sometimes break and the molecules then mix with the molecules in the liquid - forming a solution. This is called dissolving.
What is a solute?
the substance being dissolved
What is a solvent?
The liquid the solute is dissolving into
What is a saturated solution?
A solution, where the maximum amount of solute has been dissolved, so no more solute will dissolve in the solution