Unit 2 – Atoms, Molecules and Ions
1/49
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
50 Terms
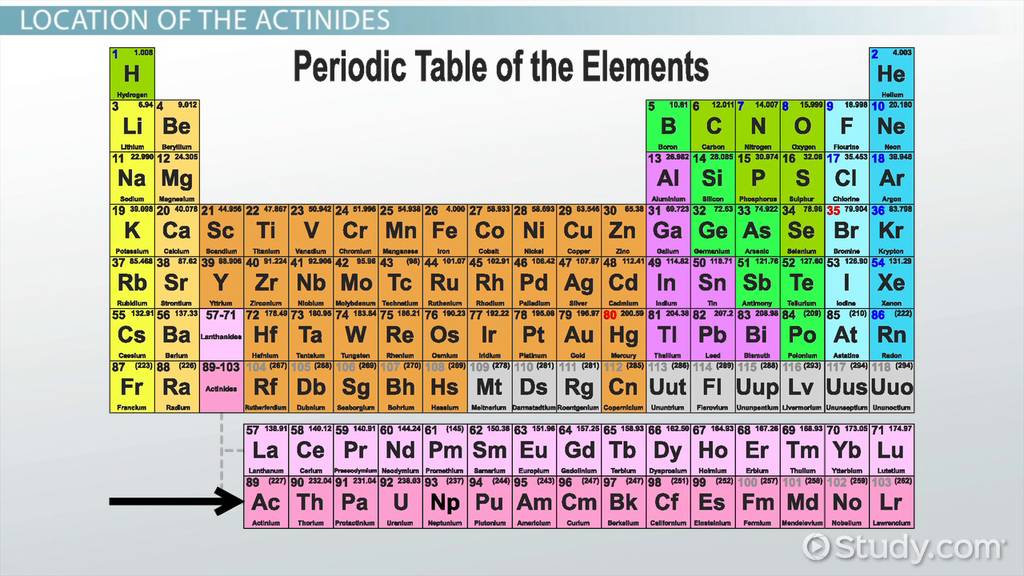
Actinide
Metals in the bottom row of the periodic table (inner transition metals)
example) Uranium
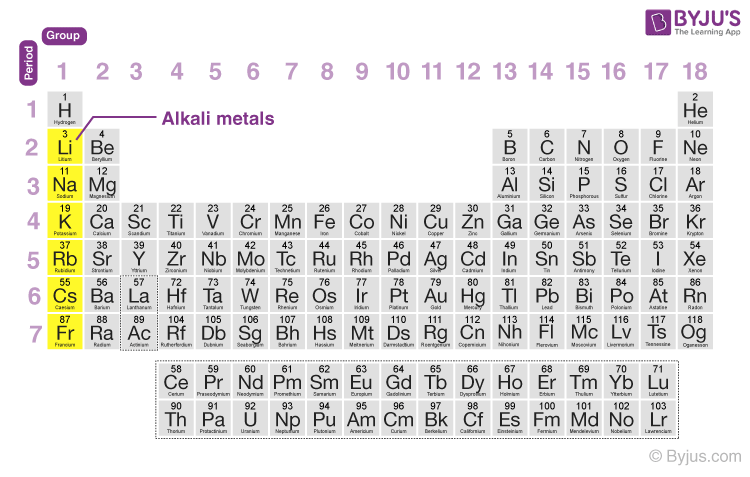
Alkali Metal
Elements in group 1 of the periodic table, very reactive
example) Sodium (Na)
Alkaline Earth Metal
Elements in group 2 of the periodic table, less reactive than alkali metals
example) Calcium (Ca)
Alpha Particle (α particle)
A particle with 2 protons and 2 neutrons, like a helium nucleus
example) Released in radioactive decay
Anion
Negatively charged atom or molecule with more electrons than protons
example) Cl⁻
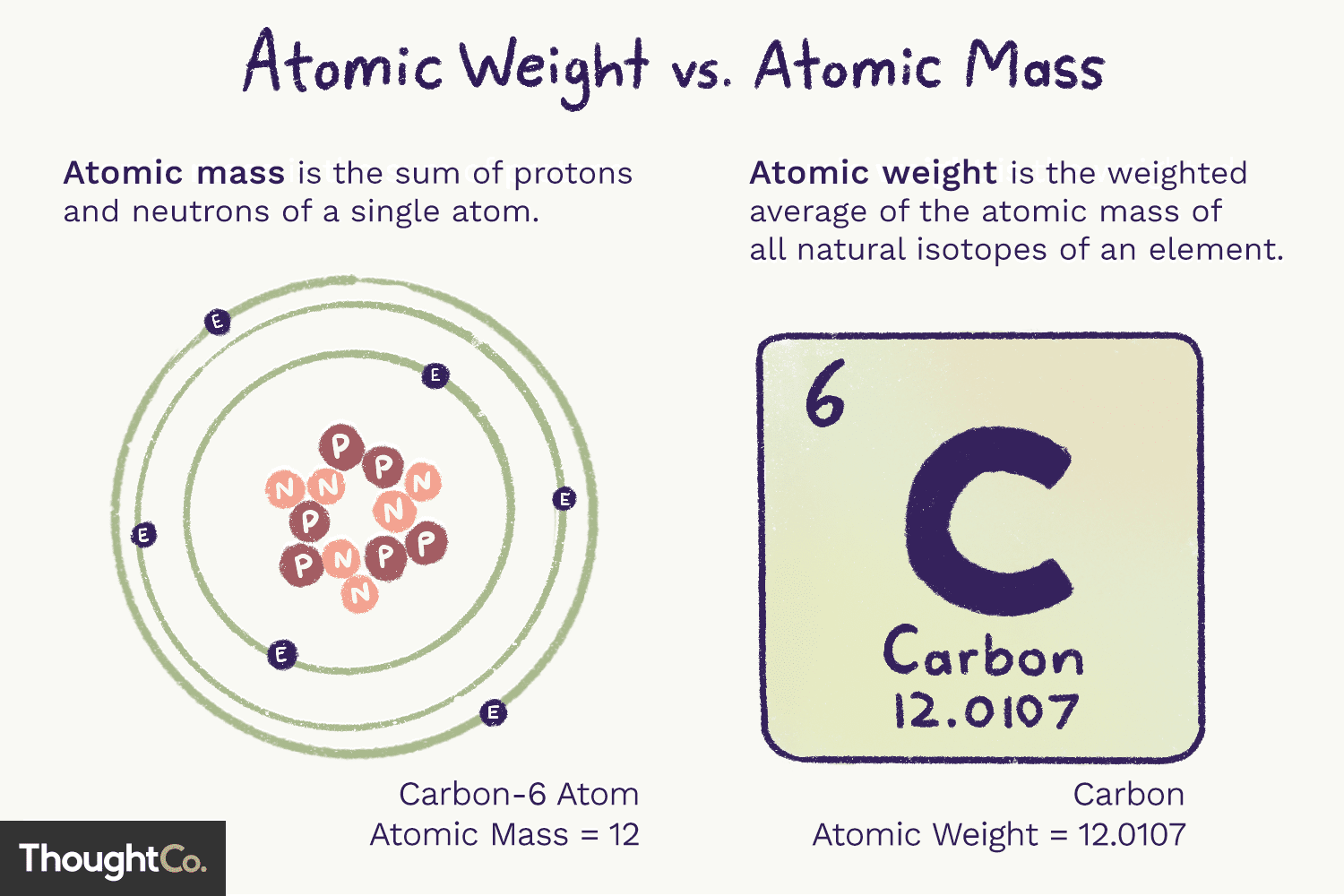
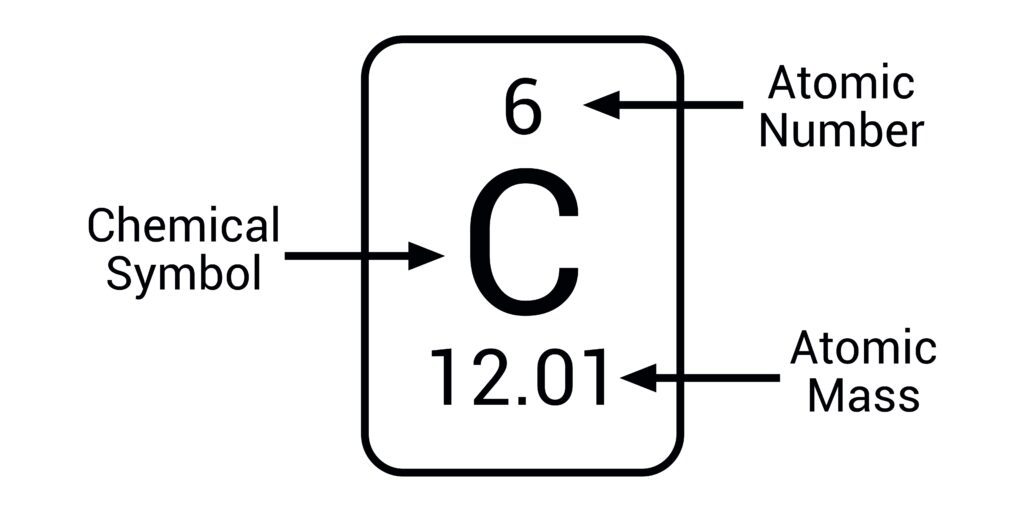
Atomic Mass
Average mass of atoms of an element, measured in amu
example) Carbon ≈ 12.01 amu
Atomic Mass Unit (amu or Dalton, Da)
Unit of mass equal to 1/12 the mass of a carbon-12 atom
example) 1 amu ≈ mass of 1 proton or neutron
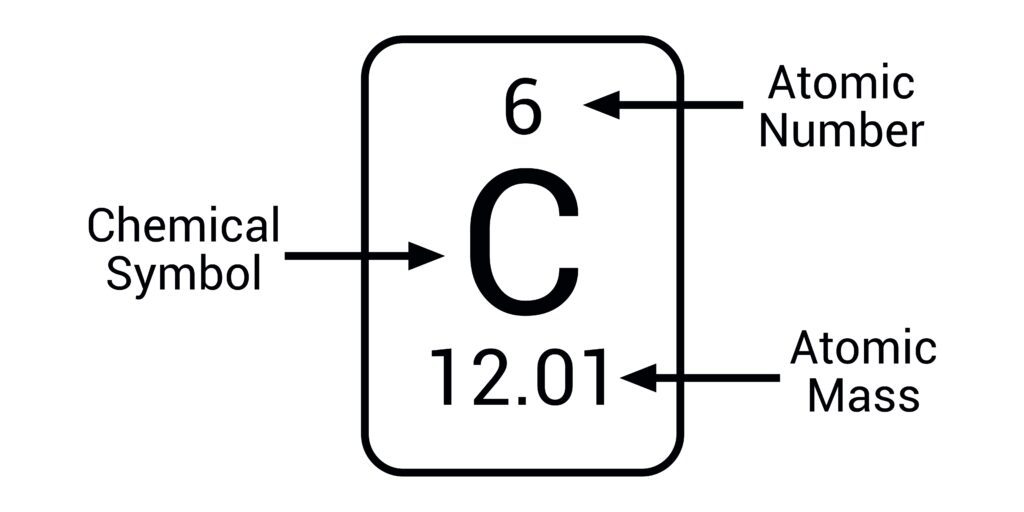
Atomic Number (Z)
Number of protons in the nucleus of an atom, defines the element
example) Oxygen = 8
Binary Acid
Acid made of hydrogen and one other element, releases H⁺ in water
example) HCl
Binary Compound
Compound with two different elements example) NaCl
Cation
Positively charged atom or molecule with fewer electrons than protons
example) Na⁺
Chalcogen
Elements in group 16
example) Oxygen (O), Sulfur (S)
Chemical Symbol
One- to three-letter abbreviation for an element
example) H for hydrogen, Fe for iron
Covalent Bond
Bond where atoms share electrons
example) The O–H bonds in water
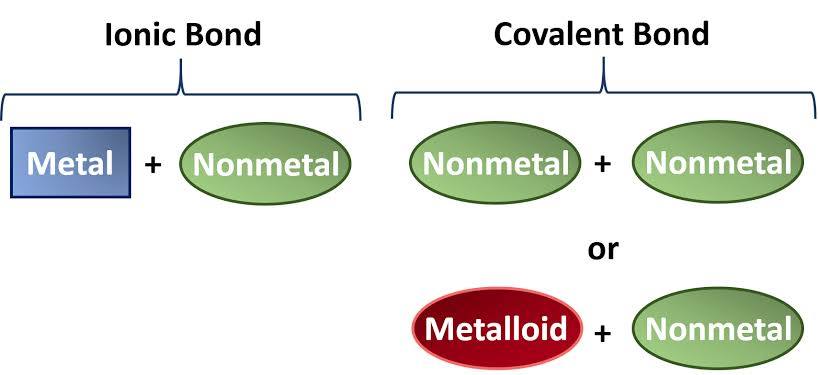
Covalent Compound (Molecular Compound)
Compound made of atoms held together by covalent bonds
2 or more non metal
example) CO₂
Dalton (Da)
Alternative name for the atomic mass unit
example) 1 Da = 1 amu
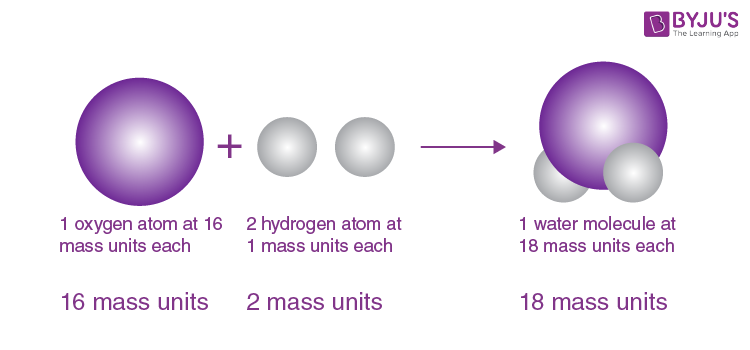
Dalton’s Atomic Theory
Early theory saying matter is made of atoms, atoms of one element are the same, and compounds form in whole-number ratios
example) Explains why water is always H₂O
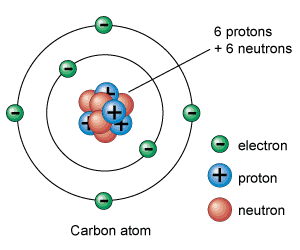
Electron
Negatively charged particle outside the nucleus, very small mass
example) Found orbiting around the nucleus
Empirical Formula
Simplest whole-number ratio of atoms in a compound
example) H₂O₂ → HO
Fundamental Unit of Charge
The charge of one electron (e = 1.602 × 10⁻¹⁹ C)
Group
Vertical column in the periodic table
Halogen
Elements in group 17, very reactive
example) Fluorine (F)
Hydrate
Compound with water molecules attached inside its crystals
example) CuSO₄·5H₂O
Inert Gas (Noble Gas)
Elements in group 18, very unreactive
example) Neon (Ne)
Inner Transition Metal
Elements in the two bottom rows of the periodic table, includes lanthanides and actinides
example) Uranium or Cerium
Ion
Atom or molecule with a positive or negative charge from losing or gaining electrons
example) Na⁺, Cl⁻
Ionic Bond
Attraction between oppositely charged ions example) Na⁺ and Cl⁻ in NaCl
Ionic Compound
Compound made of cations and anions in fixed ratios
Opposite sides of the periodic table (metal+nonmetals)
example) NaCl
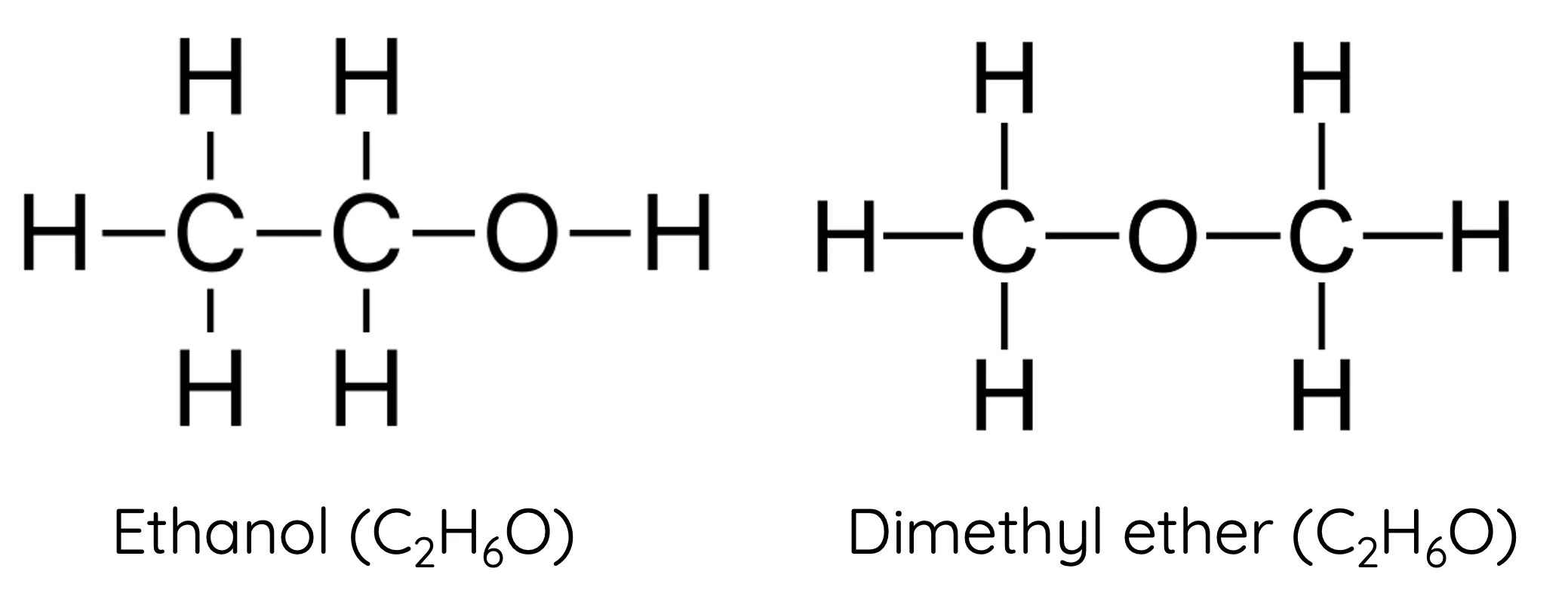
Isomers
Compounds with the same chemical formula but different structures
example) Butane and isobutane
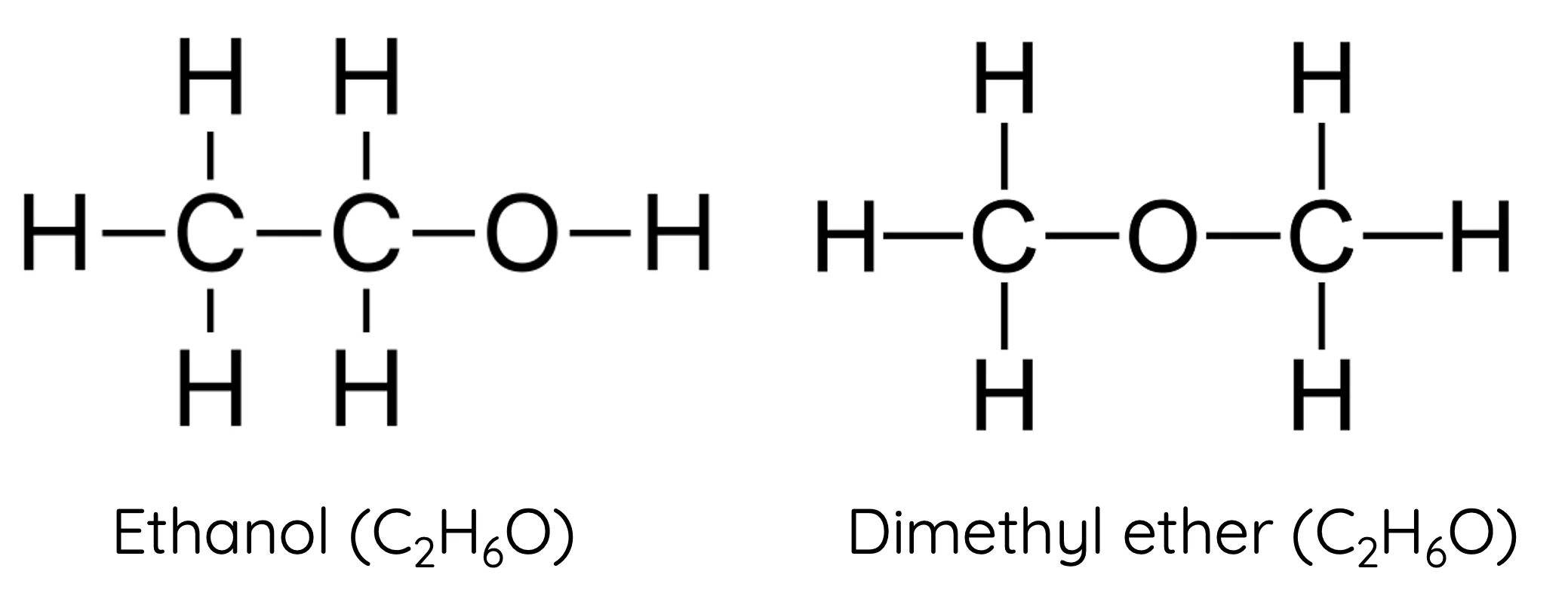
Isotopes
Atoms of the same element with different numbers of neutrons
example) Carbon-12 and Carbon-14
Lanthanide
Metals in the top row of the bottom section of the periodic table (inner transition)
example) Cerium (Ce)
Law of Constant Composition (Law of Definite Proportions)
A pure compound always has the same elements in the same ratios
example) Water is always H₂O
Main-Group Element (Representative Element)
Elements in groups 1, 2, and 13–18 example) Sodium (Na), Oxygen (O)
Mass Number
Total number of protons and neutrons in the nucleus
example) Carbon-14 has 14
Metal
Shiny, bendable, good conductor of heat and electricity
example) Copper (Cu)
Metalloid
Element with some properties of metals and some of nonmetals
example) Silicon (Si)
Molecular Compound (Covalent Compound)
Compound of atoms joined by covalent bonds
example) H₂O
Molecular Formula
Shows the exact number of atoms of each element in a molecule
example) C₆H₁₂O₆
Neutron
Neutral particle in the nucleus of an atom
Noble Gas (Inert Gas)
Elements in group 18, unreactive
example) Helium (He)
Oxyacid
Acid with hydrogen, oxygen, and another element
example) H₂SO₄
Oxyanion
Negatively charged ion made of oxygen and another
element example) NO₃⁻
Period (Series)
Horizontal row in the periodic table example) Period 2 has Li, Be, B, C, N, O, F, Ne
Periodic Law
Properties of elements repeat in a pattern based on atomic number
example) Explains periodic trends like reactivity
Pnictogen
Elements in group 15 example) Nitrogen (N), Phosphorus (P)
Polyatomic Ion
Ion made of more than one atom
example) SO₄²⁻
Proton
Positively charged particle in the nucleus
example) Hydrogen atom has 1 proton
Structural Formula
Shows which atoms are in a molecule and how they are connected example) H–O–H for water
Transition Metal
Elements in groups 3–12
example) Iron (Fe)
Unified Atomic Mass Unit (u)
Another name for the atomic mass unit (amu)
example) 1 u = 1 amu