Biology - Topic 2: enzymes
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
What are enzymes? (not function)
complex, 3D, gobular proteins with a specific tertiary shape
What bonds form the overall 3D shape of an enzyme?
> hydrogen
> ionic
> disulfide bridges
> hyrophilic and hydrophobic
Metabolism?
the chemical reactions of an organism
Two types of metabolic reactions?
breaking down and building up
Anabolic reaction?
larger molecules are built up from smaller ones
Examples of an anabolic reaction?
the synthesis of proteins
Catabolic reaction?
larger molecules are broken down into smaller ones
Example of a catabolic reaction?
the digestion of starch into sugar by amylase
How do enzymes work?
> The substrate fits into the enzyme's active site which forms the enzyme-substrate complex
> Bonds form between some of the amino acids in the enzyme and substrate
> The substrate becomes an enzyme-product complex so that the products no longer fit in the active site and are released
Activation energy?
The energy required to initially break bonds
How do enzymes speed up the rate of reaction?
They lower the activation energy
Graph for enzyme energy barrier
page 5
What is the active site?
the part of the enzyme molecule to which the substrate binds
How does the active site work?
> The active site is where certain amino R-groups are exposed
> it is complementary to the substrate
> the substrate fits into the active site and interacts with these R-groups of amino acids by ionic and hydroge bonding
> this forms the enzyme substrate complex
Enzyme specifity?
one type of substrate can be catalysed by only one type of enzyme
Lock and key model?
the shape of the enzyme's active site is complementary to the shape of the substrate. They fit together like a lock and key
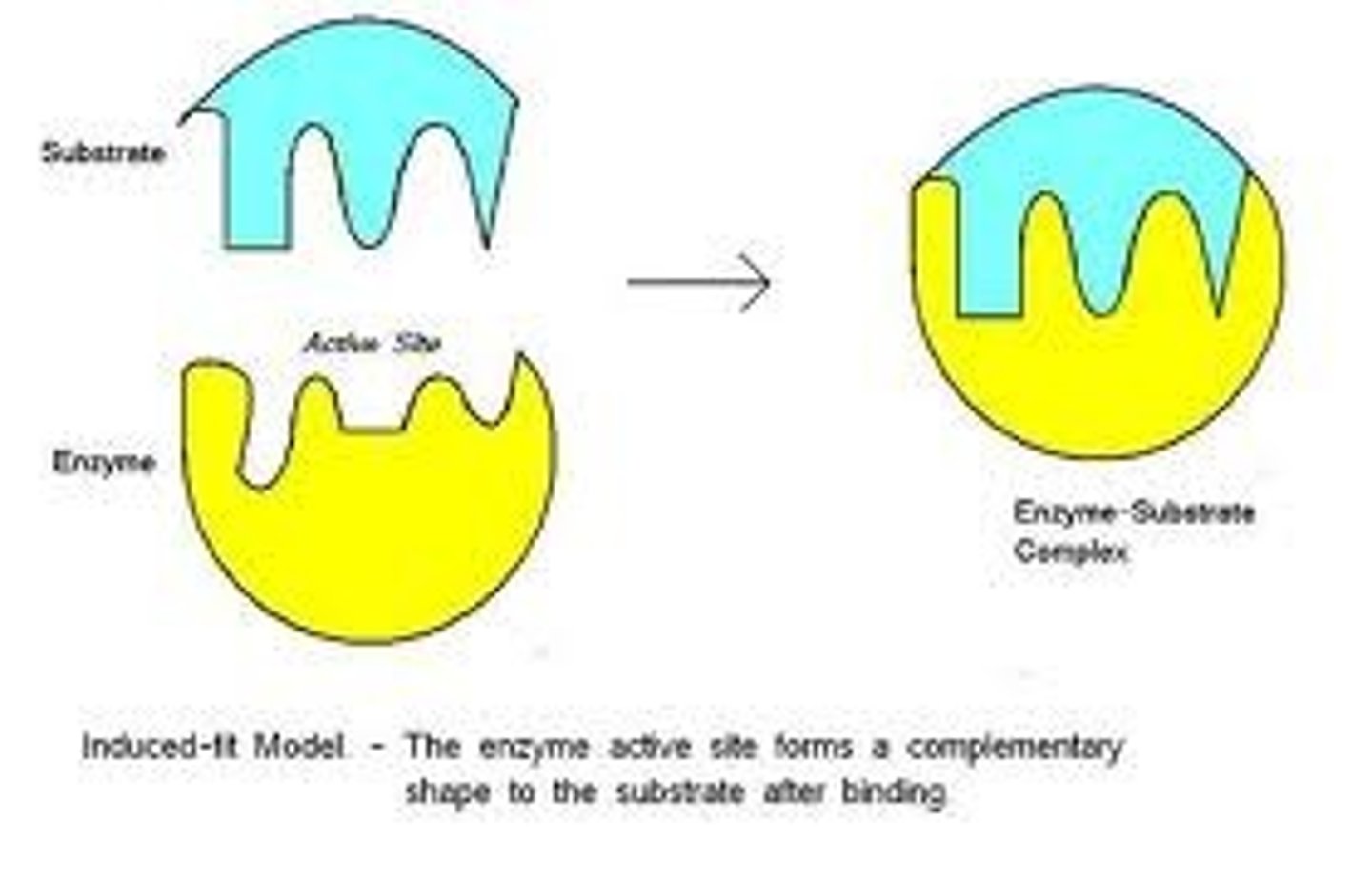
Induced fit model?
The active site of the enzyme very closely matches the shape of the substrate but can mould itself around the substrate, forming a precise fit
> the active site is flexible
What are cofactors?
non-protein substances that some enzymes require in order to function
Examples of cofactors?(3)
> metal ions
> prosthetic groups
> coenzymes
How do metal ions act as cofactors?
they form attachments to the enzyme changing the shape of the active site, enabling a reaction to take place
Examples of metal ion cofactors?
Mg2+, Ca2+, Fe3+
Example of a prosthetic group?
Haem in catalase
What are coenzymes?
another type of cofactor which are non-protein organic molecules that aren't permanently attached
Examples of coenzymes?
> NAD
> FAD
What factors effect the activity of enzymes?(5)
> Temperature
> pH
> concentration of enzyme
> concentration of substrate
> presence of inhibitors
Graph for the effect of temperature on enzymes?
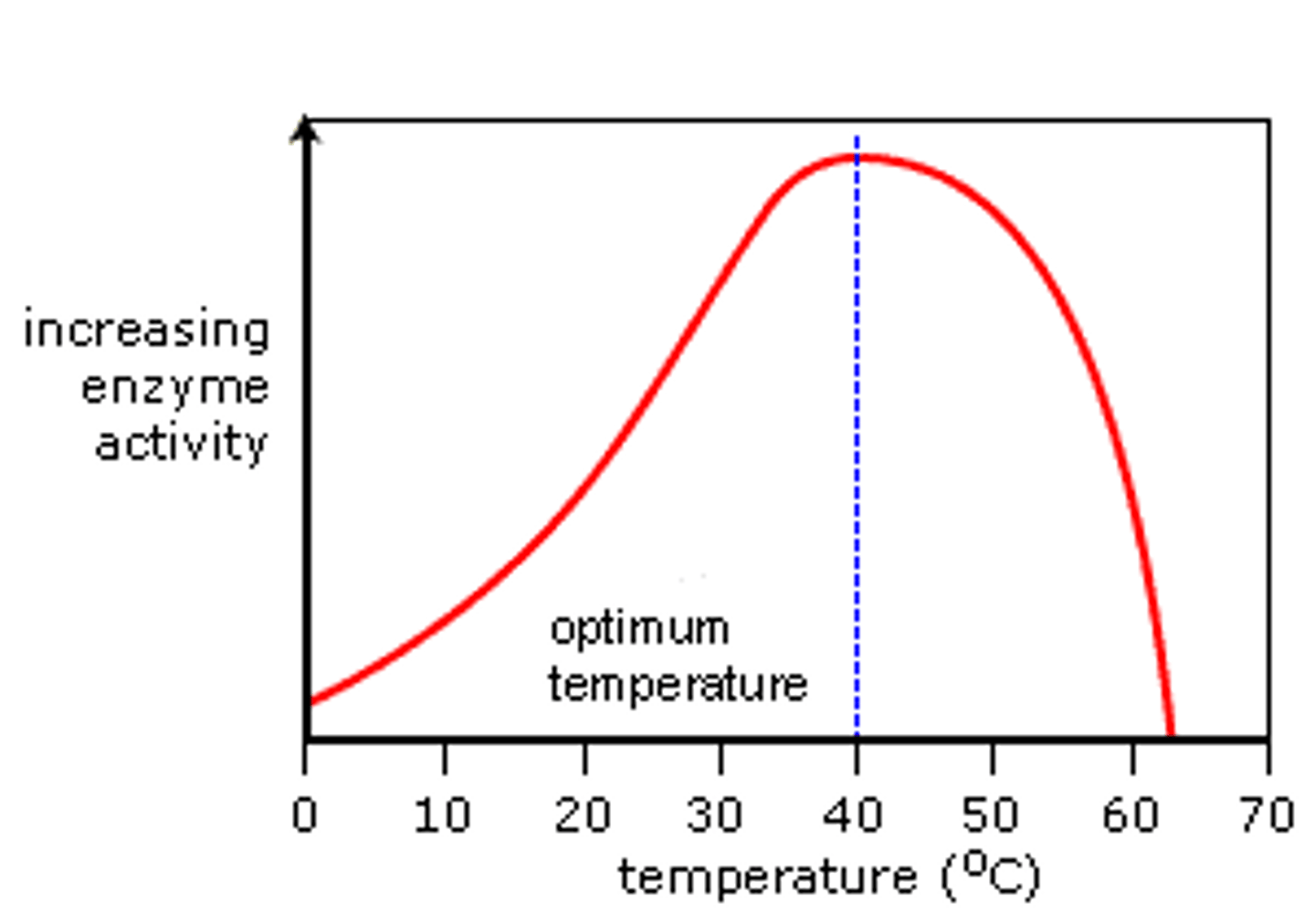
Description of enzyme and temperature graph?
> As temperature increases, the rate of reaction increases
> once the optimum temperature is exceeded the rate of reaction decreases rapidly
Explanation of enzyme and temperature graph?
> As temperature increases the molecules gain more kinetic energy and therefor more faster
> There are more successful collisions and more enzyme-substrate complexes are formed
> more products will be made
> once the optimum is exceeded the high temps break the hydrogen bonds in the tertiary structure of the enzyme which alters the shape of the active site and stops substrates from entering and being broken down
Graph of effect of pH on enzyme activity?
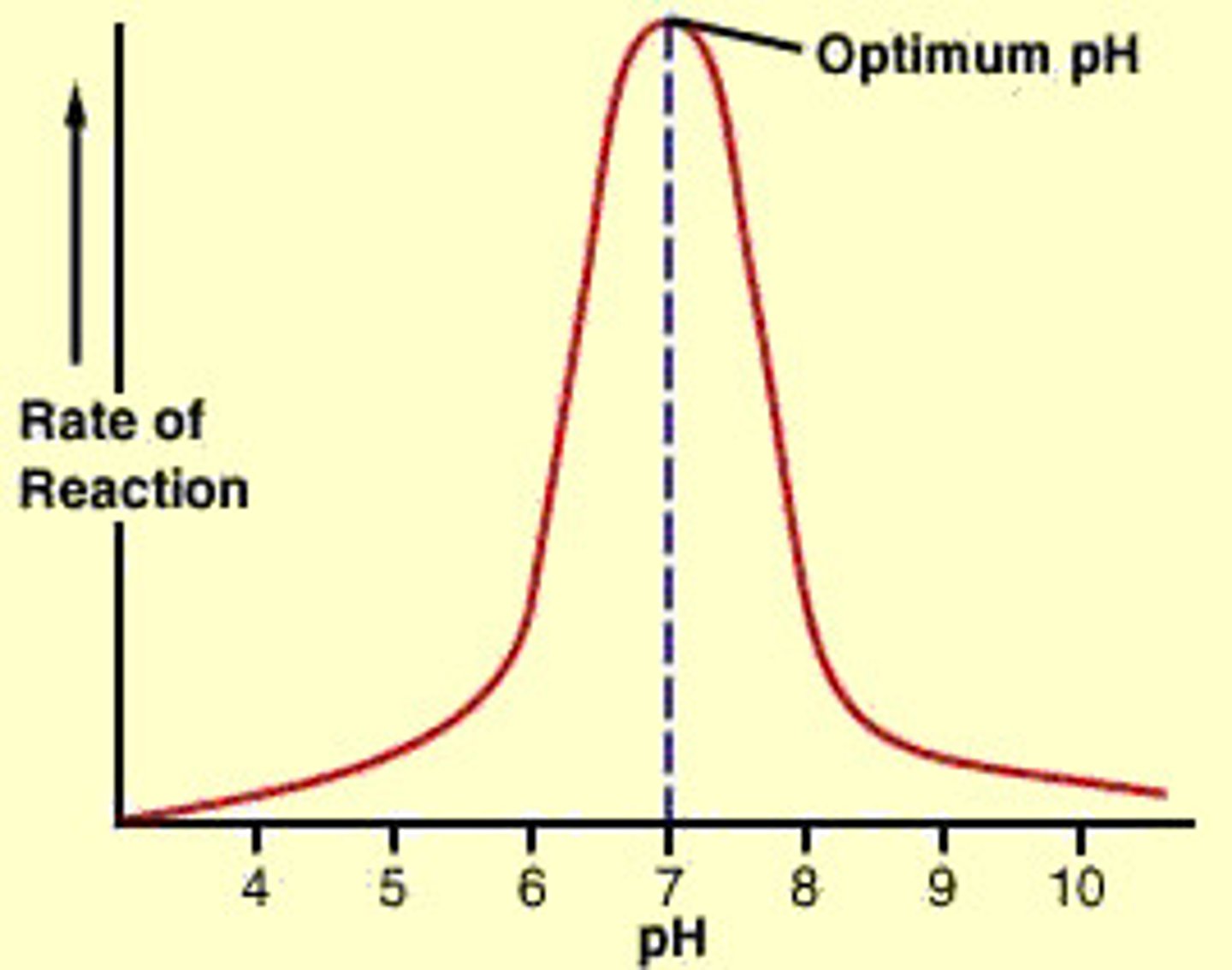
Description of the effect of pH on enzyme activity?
> As the pH increases the rate of reaction increases until the optimum pH is reached
> once the optimum pH is exceeded the rate of reaction will decrease at the same rate
Explanation for the effect of pH on enzyme activity?
> As the pH deviates from the optimum, the ionic bonds within the tertiary structure are broken which alters the shape of the enzyme's active site and doesn't allow substrates to fit and be broken down
> less products will form
Graph for the effect of enzyme concentration on enzyme activity?
page 19
Description of the graph for the effect of enzyme conc on rate of reaction?
> As the enzyme conc increases, so does the rate of reaction
> the graph eventually levels off
Explanation for the effect of enzyme conc on rate of reaction?
> At low enzyme conc there is great competition for the active sites so rate of reaction is low
> As the enzyme conc increases more enzyme active sites become available to form enzyme-substrate complexes
> more reactions can take place and more products will form
> the graph levels off when the substrate molecules become the limiting reactant
Graph for the effect of substrate concentration on rate of reaction?
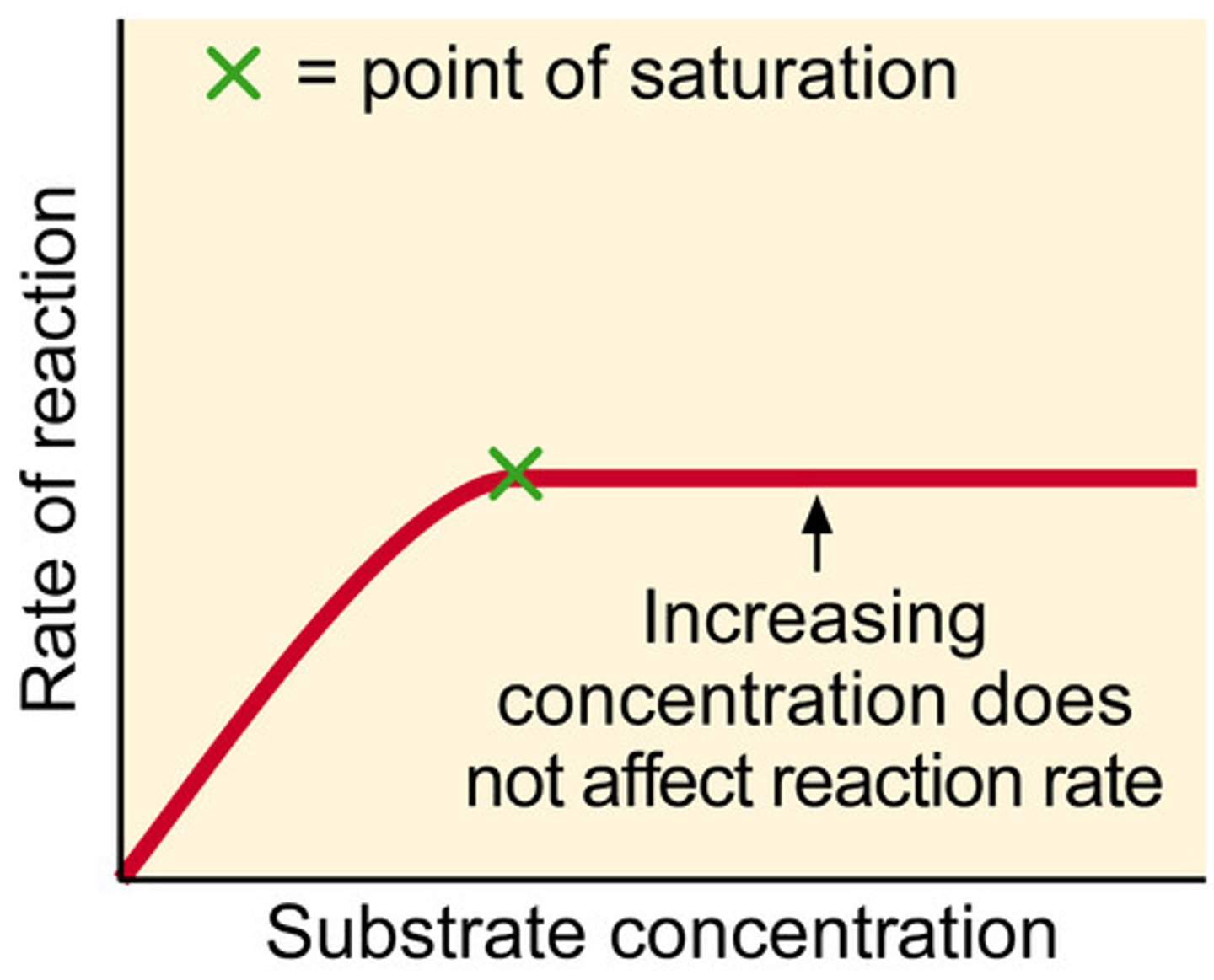
Description of graph for the effect of substrate concentration on rate of reaction?
> As substrate con increases, the rate of reaction increases
> the graph eventually levels off
Explanation for the graph for the effect of substrate concentration on rate of reaction?
> At low substrate conc the rate of reaction is low because the substrates occupying all the active sites they can but there are still some unoccupied
> substrate conc is the limiting reactant
> the graph eventually levels off because all of the enzyme's active sites are in use
> enzyme conc becomes the limitng reactant
Name for the point at which the graph for substrate conc levels off?
point of saturation
What are inhibitors?
substances that prevent enzymes from forming enzyme-substrate complexes and so stop or slow down the catalysis of the reaction
How does the inhibitor affect the rate of reaction?
> the substrate can't bind to the active site
> no enzyme substrate complexes can form
> so no product is formed
> rate of reaction decreases or is zero
Two types of inhibitors?
> competitive
> non-competitive
How do competitive inhibitors work?
> molecules that are complementary to all or part of the active site and compete with the substrate for the active site
> blocks the active site so substrate can't enter and form enzyme-substrate complex
Graph for the effect of a competitive inhibitor on substrate concentration?
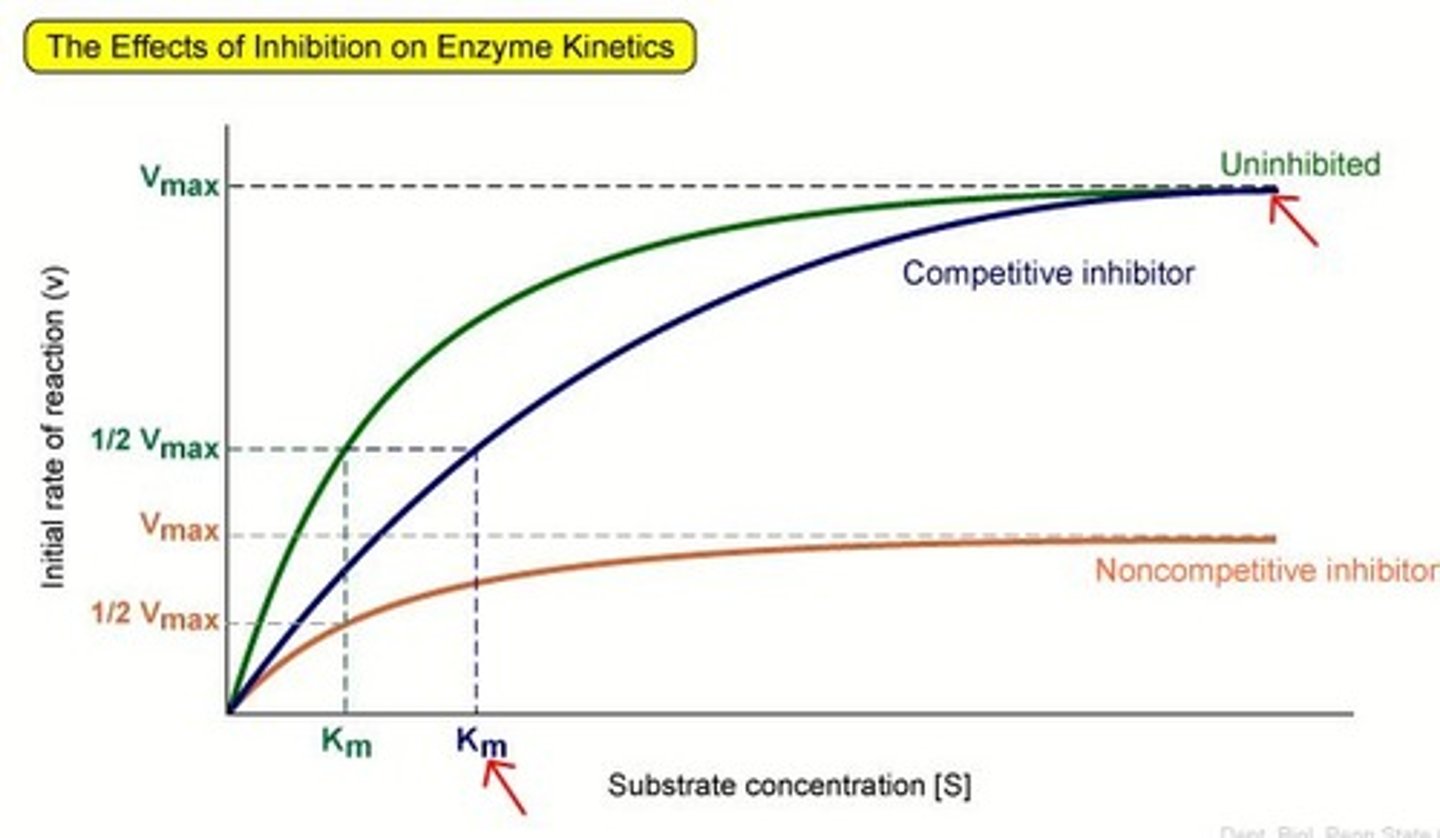
How to reduce the effect of a competitive inhibitor?
increase substrate concentration
How does a non-competitive inhibitor work?
the inhibitor binds to the enzyme at the allosteric site and changes the shape of the active site or blocks in irreversibly so the substrate is no longer complementary
Effect of increasing substrate concentration when non-competitive inhibitors are present?
increase in substrate concentration doesn't decrease the effect of the inhibitors
Graph for the effect of non-competitive inhibitors on substrate concentration?
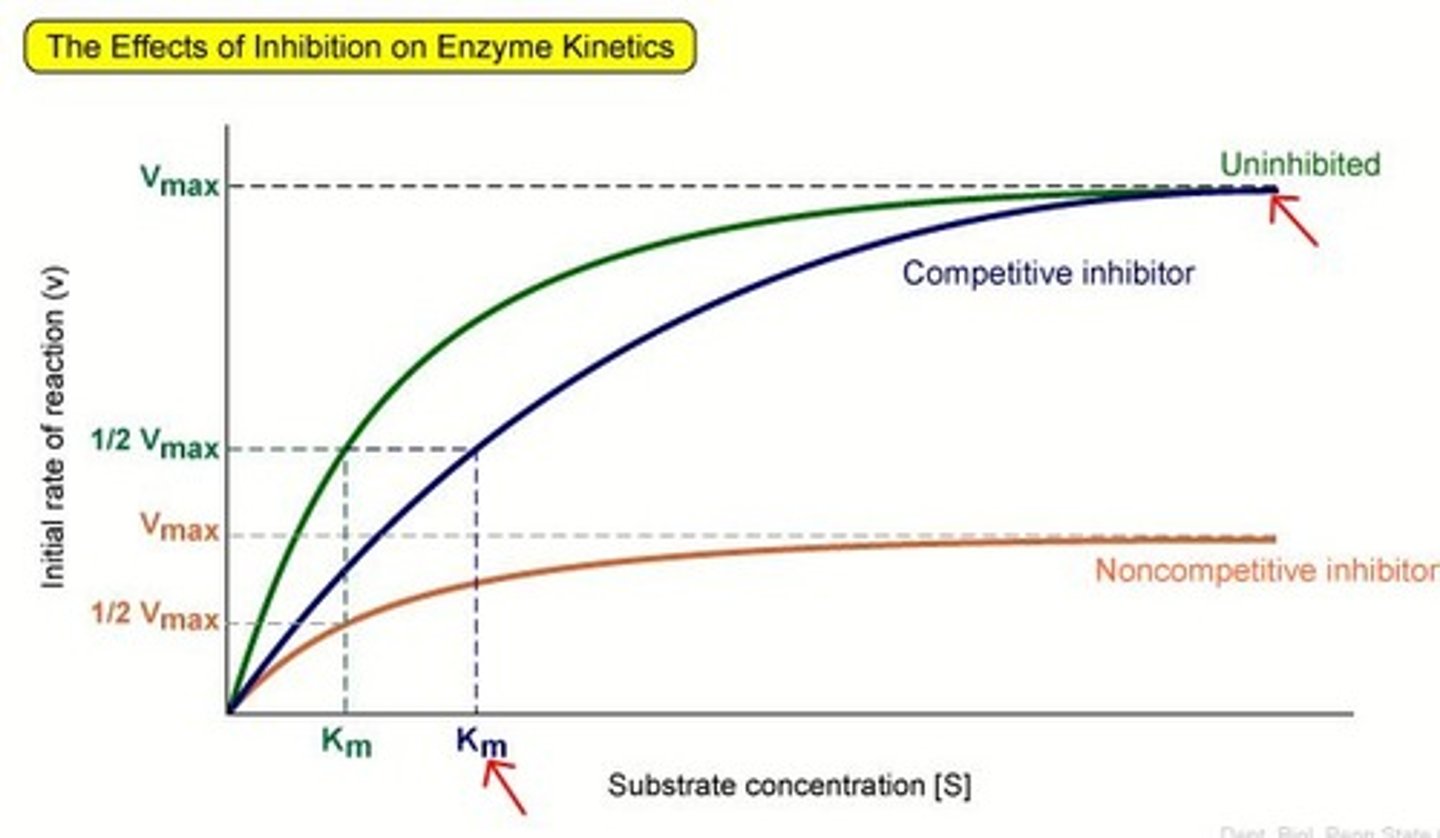
Are inhibitors reversible?
many inhibitors are reversible but some permanently damage the active site by breaking the bonds in the tertiary structure
How are enzymes used as biomarkers of disease?
Some diseases cause the presence of enzymes so they can be used as diagnostic tools or to track the progression of a disease
Where will these enzymes caused by diseases be found?
> blood
> urine
> sputum
Example of an enzyme produced because of illness?
Elastase
When is elastase produced?
Released by white blood cells due to lung infection
Advantage of elastase?
Elastase breaks down bacterial pathogens
Disadvantage of elastase?
Breaks down the protein elastin which contributes to the elasticity of the alveoli
Inhibitor for elastase?
Alpha-1-antitrypsin (A1AT) which prevents elastase activity after the infection has been cleared
How can cigarette smoke effect elastase?
> cigarette smoke increases production of elastase
> smoke inhibits production of A1AT
> elastin in alveoli is broken down
Lung disease cause by elastase?
elastase-induced emphysema
How can enzyme inhibitors work as therapeutic drugs?
enzyme inhibitors can target enzymes which are causing diseases so the progression of the disease can be slowed
Requirements for enzyme inhibitors to be effective?
> specific so only works on target enzyme
> works at low doses so no inhibitor build up as toxic
Examples of inhibitors used as therapeutic drugs?(4)
> A1AT - reduces harmful effects of lung infection
> ACE - treat high BP by stopping enzymes that constrict the blood vessels
> Penicillin - inhibits enzymes that create bacterial cell walls
> Anti viral drugs - inhibit DNA/RNA polymerase
Current research on inhibitors as therapeutic drugs?
trying to work out shape of active site of certain disease causing enzymes so complementary inhibitors can be created
How to maximise efficiency of enzymes?
Immobilise them
What is immobilization of enzymes?
Attaching enzymes or putting inside an insoluble support
Methods of immobilization?(4)
> Adsorption
> Cross linkage
> Entrapment
> Encapsulation
Adsorption?
the enzymes are attached by weak forces to an inert substance such as glass or a matrix
Cross linkage?
The enzymes are bonded covalently to a matrix, such a cellulose, as a consequence of chemical reactions
Entrapment?
the enzymes are trapped within polymers such as alginate beads or microspheres
Encapsulation?
The enzymes are trapped inside a selectively permeable membrane
Advantages of immobilising enzymes?(5)
> thermostable
> more resistant to wider pH range
> enzymes can be retained and reused: cheaper
> product is enzyme free which simplifies downstreaming, reduces purification costs and avoids possible allergic reaction
> commercial processes can be continuous: faster + less waste
Disadvantage of immobilising enzymes?
enzyme activity reduced bcs not all active sites are available and it takes time for substrate to diffuse
Why are enzymes very effective biosensors?
> specific
> quantitative
How do biosensors work?
molecule being monitored attaches to immobilized enzymes in reaction causing colour change or is converted to an electrical signal
Example of biosensors?
> clinistix
> digital blood glucose monitors
How to pregnancy tests work?
the antibody in the test strip reacts with specific proteins(hormones) to give a colour change due to antibody-enzyme complexes forming
How are enzyme inhibitors used in diagnostic reagent strips?
inhibitors are attached to the strip and if the enzyme attaches to the inhibitor a colour change will occur
> currently used to test for early cardiovascuclar disease and pre-eclampsia