research methods
1/136
Earn XP
Description and Tags
Research Methods
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
137 Terms
list experimental techniques
laboratory experiments
field experiments
natural experiments
quasi experiments
list non-experimental techniques
correlation
observations
interviews
questionnaires
case studies
content analysis
thematic analysis
define the key term ‘validity’
refers to how ‘real’ or accurate something is
its an important issue that runs throughout the whole of psychology
define the key term ‘reliability’
a measure of consistency
if the results are not consistent- then the measure is not reliable
define the key term ‘Ethics’
The set of moral principles that psychologists must follow to protect participants in research, ensuring they are treated safely, fairly, and with respect.
statistical analysis
the use of descriptive statistics, which summarise data and
inferential statistics- which tell us if the results are likely to be due to a chance or not ( based on probability)
define the term ‘ independent variable’
the variable that the researcher manipulates → which is assumed to have a direct effect on the dependent variable (DV)
define the term ‘ dependent variable’
the variable that the research measures → the variable is affected by changes in the independent variable
how many conditions does the independent variable have?
2 conditions
operationalisation
where you clearly specify/define observable behaviours + how it’ll be measured so that someone else can replicate it exactly
aim
A general statement about the purpose of a study
Hypothesis
A precise and testable statement about the relationship between the variables to be studied.
Stated at the start of a study.
two types of hypothesis
H1 – Research/alternative/experimental hypothesis
H0 – Null hypothesis - useful to know about for Inferential statistics and falsifiability)
Directional hypothesis
states the direction of the difference or relationship. Used when there is previous research to suggest a direction
non-directional hypothesis
predicts that a difference will exist between two or more variables without predicting the exact direction of the difference.
This is usually because previous research has been inconclusive, and the specific nature (direction) of the effect of the IV on the DV cannot be predicted confidently
Null Hypothesis
all investigations have a null hypothesis which suggests that any difference or relationship found is due to chance.
sample
A group of people that are drawn from the target population to take part in a research investigation
Target population
The group that the researchers draw the sample from and want to be able to generalise the findings to
usually too large to study in its entirety, so sampling techniques are used to choose a representative (typical) sample
Representative sample
A sample that closely matches the target population as a whole in terms of key variables and characteristics
Generalise
Applying results to a wider population beyond the research participants
sampling bias
when a sample is compromised of one particular type of person
five common types of sampling
· Random
· Systematic
· Stratified
· Opportunity
· Volunteer
random sampling
Every member of the target population has an equal chance of being selected.
Requires a complete list of the population.
Participants are chosen using:
Random number generator
Names from a hat
random sampling strengths
Reduces researcher bias
Likely to produce a representative sample
Allows better generalisation
random sampling limitations
Time-consuming and impractical
Some selected participants may refuse → becomes closer to a volunteer sample
Systematic Sampling
Participants are selected using a fixed interval (e.g. every 10th person).
Requires a list of the population.
Systematic Sampling strengths
Reduces researcher bias
Simple and efficient
Systematic Sampling limitations
Not fully random
Can be biased if the list has a pattern (e.g. every 10th person shares a characteristic)
May reduce representativeness
Stratified Sampling
The population is divided into subgroups (strata) based on characteristics (e.g. gender, job role).
Participants are selected in proportion to their representation in the population.
Selection within each stratum is random.
Stratified Sampling strengths
Most representative sampling method
Reduces researcher bias
Improves generalisation
Stratified Sampling limitations
Time-consuming
Difficult to identify all relevant strata
Still cannot account for all individual difference
Opportunity Sampling
Participants are chosen because they are available and willing.
Commonly used due to convenience.
Opportunity Sampling strengths
Quick and cheap
Easy to carry out
Opportunity Sampling limitations
High risk of researcher bias
Sample is often unrepresentative
Limits generalisation
Volunteer Sampling
Participants self-select by responding to an advert.
Researcher does not approach participants directly.
Volunteer Sampling strengths
Minimal effort for researcher
Ethical (participants choose to take part
Volunteer Sampling limitations
Volunteer bias – certain personality types more likely to volunteer
Often unrepresentative
Reduced generalisability
Bias
Occurs when some members of the population are more likely to be selected than others.
More common in opportunity and volunteer sampling.
Generalisation
Ability to apply findings from the sample to the target population.
More valid with random and stratified samples.
Experimental Design
Experimental design refers to how participants are allocated to the different conditions of an experiment.
what does experimental design affect
The design chosen affects:
Control of participant variables
Risk of order effects
Cost, time and validity of the research
Participant variables
Individual differences between participants (e.g. age, IQ, personality) that may affect the DV.
Order effects
Changes in performance due to the order of conditions (e.g. practice or fatigue).
Random allocation:
Assigning participants to conditions by chance to control participant variables.
Demand characteristics:
Cues that reveal the aim of the study and alter participants’ behaviour
Counterbalancing
Technique used in repeated measures to reduce order effects by varying condition order.
Independent Groups Design
Different participants are used in each condition.
Participants are allocated using random allocation.
Each participant takes part in one condition only.
Independent Groups Design strengths
No order effects (participants only complete one condition)
Reduced risk of fatigue or practice
Lower risk of demand characteristics
Independent Groups Design limitations
Requires more participants (time-consuming and costly)
Participant variables may affect results (e.g. IQ differences)
Reduces internal validity if groups are not equivalent
Repeated Measures Design
Same participants take part in all conditions.
Participants act as their own control.
Repeated Measures Design strengths
Fewer participants required
Controls participant variables
More sensitive comparison between conditions
Repeated Measures Design limitations
Order effects (practice, boredom, fatigue)
Increased risk of demand characteristics
How to control order effects :Counterbalancing
Half the participants complete condition A then B
Other half complete condition B then A
Order effects are spread evenly, not eliminated
Matched Pairs Design
Participants are paired based on key characteristics (e.g. age, IQ).
One participant from each pair is placed in each condition.
Treated like independent groups in analysis.
Matched Pairs Design strengths
Reduces participant variables
No order effects
More valid comparison than independent groups
Matched Pairs Design limitations
Very time-consuming
Difficult to match participants accurately
Impossible to control all individual differences
Extraneous variables
the variables that could affect the DV. These are essentially nuisance variables that would want to minimise and therefore CONTROL. Does not vary systematically with the IV.
Confounding variables
Where extraneous variables are important enough to cause a change in the DV, they become confounding variables.
Confounding Variables are any variable (other than the IV) that could affect the DV. CVs change systematically with the IV
Situational Variables
Factors connected to the research situation that could affect the DV e.g. temperature, noise, lighting, distractions
Participant Variables:
Factors connected to the participant that could affect the DV e.g. IQ, age, experience, motivation
Investigator Effects
occur when the researcher’s behaviour (conscious or unconscious) influences the DV.
This can happen through:
Study design
Selection of participants
Interaction with participants
what can an investigator effect
Researchers may unintentionally give cues about the aim or expected behaviour, affecting participant responses.
Investigator effects can also affect observations, as researchers may interpret behaviour differently.
The personal characteristics of the investigator (e.g. age, gender, accent, status) can influence participants’ behaviour.
Investigator effects reduce the validity of the findings.
Control of Investigator Effects
Controlled using a double-blind procedure.
Neither the researcher nor participants know the aim of the study or the IV.
Prevents the researcher from influencing participants.
Common in drug trials comparing a drug with a placebo
Demand characteristics
cues from the research situation that allow participants to guess the aim of the study.
This leads to participant reactivity, where behaviour changes.
Participants may:
Act to support the hypothesis
Act against it (“screw-you” effect)
This reduces the validity of the results as behaviour is unnatural.
Control of Demand Characteristics
Controlled using a single-blind procedure.
Participants do not know the aim or IV, but the researcher does.
Often involves deception.
Common in studies comparing a drug and a placebo.
Systematic Error
A consistent bias in a study that affects results in the same way each time.
It occurs due to flaws in the design or procedure (e.g. faulty equipment, leading questions).
This reduces the validity of the results because the findings are consistently inaccurate.
random error
Unpredictable, chance variations that affect results differently each time.
Caused by uncontrolled variables (e.g. participant mood, distractions).
This reduces the reliability of results because findings are inconsistent.
Control of Variables: randomisation
The use of chance to reduce the effects of the researcher's unconscious biases when designing an investigation or a task, or allocating participants
This involves organising participants and materials in a way that is randomly generated and is free from bias
Control of Variables: standardisation
the process in which procedures used in research are kept the same
more likely that results will be replicated on subsequent occasions when research is standardised which means that data reflects a meaningful pattern and was not a one off chance result
random allocation
an extremely important process in research
gently decreases the impact of participant variables - any pp variables should be equally spread out across both conditions
both conditions are likely to be affected equally
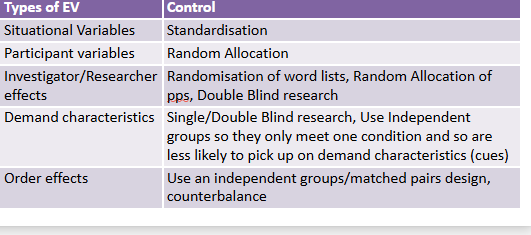
summary of controls of research
pilot study
is a small-scale trial run conducted before the main study.
Its purpose is to identify problems or confusions in the research design.
Results from the pilot study are not included in the final analysis.
It helps prevent wasting time and money by spotting issues early.
The same participants should not be used in both the pilot and main study.
what does a pilot study check?
Whether the procedure works
If instructions and debrief are clear
Whether the task is too easy or too difficult
If anything important has been missed out
It reduces the risk of participant misunderstanding, which could lead to invalid or meaningless results.
Questionnaires must be piloted to?
Ensure all questions are clear and answerable
Check reliability and validity
Overall purpose: to find and correct errors in methodology or procedure before carrying out the main investigation.
what do pilot studies improve?
Pilot studies improve validity, reliability, and ethical quality of research.
Single blind:
when the pps are not told the true aim of the research. This is an attempt to control for the confounding variable of demand characteristics.
Double blind
when the pps are not told the true aim of the research and the researcher does not know them either.
In this case, a third party researcher will conduct the experiment.
This also helps to reduce demand characteristics and investigator effects.
Experimental group
the group which have the IV altered in some way. For example, in a drug trial, this group would be given the drug.
Control Group
the group which does not have the IV altered in some way. For example, in a drug trial, this group would be given a placebo (a fake drug)
Responsibility of Psychologists
Most psychological research involves human participants.
Psychologists have a duty to protect participants’:
Psychological well-being
Physical health
Values
Dignity
Research should be conducted in an ethical manner.
What Are Ethical Issues?
Ethical issues are considerations researchers must address:
Before the research begins
During the investigation
After the study is completed
Purpose of Ethical Considerations
Ethical issues focus on:
The welfare of participants
The integrity and credibility of the research
The responsible use of data
Following ethical guidelines ensures research is safe, respectful, and valid.
Ethical Research
research that is appropriate to its circumstances and morally correct
Ethical Issues
arise when there is a conflict between the aim or intentions of the research and the rights and well-being of the participants.
Six of the main ethical guidelines
Deception
· Right to withdraw
· Informed consent
· Privacy and confidentiality
· Protection from harm
DECEPTION
When information is
deliberately withheld
from participants or
they are knowingly
misled.
WHY IS DECEPTION UNETHICAL? (IF BROKEN)
It prevents participants from giving fully informed consent which means that they might be taking part in research that goes against their views or beliefs.
HOW TO DEAL WITH THE ISSUE OF DECEPTION ? (IF BROKEN)
At the end of the study the participants should be fully debriefed and told the true aim and nature of the research. At this point the participant should be given the right to withdraw the publication of their results. The contact details of the experimenter should be given if participants have any further questions or queries
RIGHT TO WITHDRAW
Participants have the
right to withdraw
(remove themselves or
their data from the
study) at any stage.
This includes after the
research has been
conducted, in which
case the researcher
must destroy any data
or information
collected.
WHY IS RIGHT TO WITHDRAW UNETHICAL? (IF BROKEN)
Participants who are not given the right to withdraw may feel unnecessary or undue stress and are therefore not protected from harm.
HOW TO DEAL WITH THE ISSUE OF RIGHT TO WITHDRAWAL ? (IF BROKEN)
At the beginning of the study in the consent form the pp should be informed of their right to withdraw.
At the end of the study the participants should be fully debriefed and told the true aim and nature of the research.
At this point the participant should be given the right to withdraw the publication of their results.
The contact details of the experimenter should be given if participants have any further questions or queries
INFORMED CONSENT
When someone
consents to participate
in research, their
consent must be fully
informed which means
the aims of the
research should be
made clear before they
agree to participate.
WHY IS INFORMED CONSENT UNETHICAL? (IF BROKEN)
Lack of informed consent may mean that the participant is taking part in research that goes against their wishes or beliefs. It is possible that the participant may have felt obliged to take part or even coerced into it, especially if they are not fully informed.
HOW TO DEAL WITH THE ISSUE OF INFORMED CONSENT? (IF BROKEN)
Pps should be issued with a consent letter (informed consent form), that details all the relevant information that might affect their decision to participate.
If the pps agrees, they sign the form and the study goes ahead with them taking part.
If they refuse, they walk away and are not included.
what do we need for children under 16 in regards of informed consent
For children under 16, parental consent is needed. It is possible to gain consent from those in ‘loco parentis’ e.g. A teacher.
Presumptive consent:
involves taking a random sample of the population and introducing them to the research, including any deception which may result.
If they agree to take part in the research it can be presumed that other future participants would do the same so the consent is generalised.
Prior general consent
involves participants agreeing to take part beforehand in numerous psychological investigations, which may or may not involve deception.
This, in effect, means that they will have given consent for being deceived
Retrospective consent
involves participants giving consent for their participation after already taking part, for instance, if they were not aware that they were the subject of an investigation.
Children as participants: involves gaining the consent of the parent(s) in writing for children under the age of 16 to participate in any psychological research.
PRIVACY AND
CONFIDENTIALITY : privacy
is the right of
individuals to decide
how information about
them will be
communicated to
others.
PRIVACY AND
CONFIDENTIALITY : confidentiality
is
where a participant’s
personal information is
protected by law under
the Data Protection Act
both during and after
the experiment.