isomerism and carbonyl compounds
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
name the six functional groups of carbonyl compounds
•ester
•aldehide
•ketone
•carboxylic acide
•acyl chloride
•acid anhydride
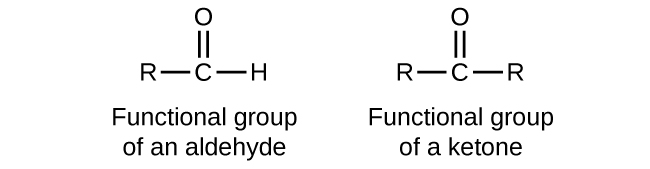
define aldehydes and ketones
compounds that both contain a carbonyl group (C=O) as their functional group, with aldehydes having the carbonyl group at the end of a carbon chain and ketones having it within the chain.
define a neucliophile
an electron-rich species that donates a pair of electrons to form a chemical bond with an electron deficient species.
define electrophile
an electrophile is an electron acceptor species that accepts electrons to form a chemical bond.
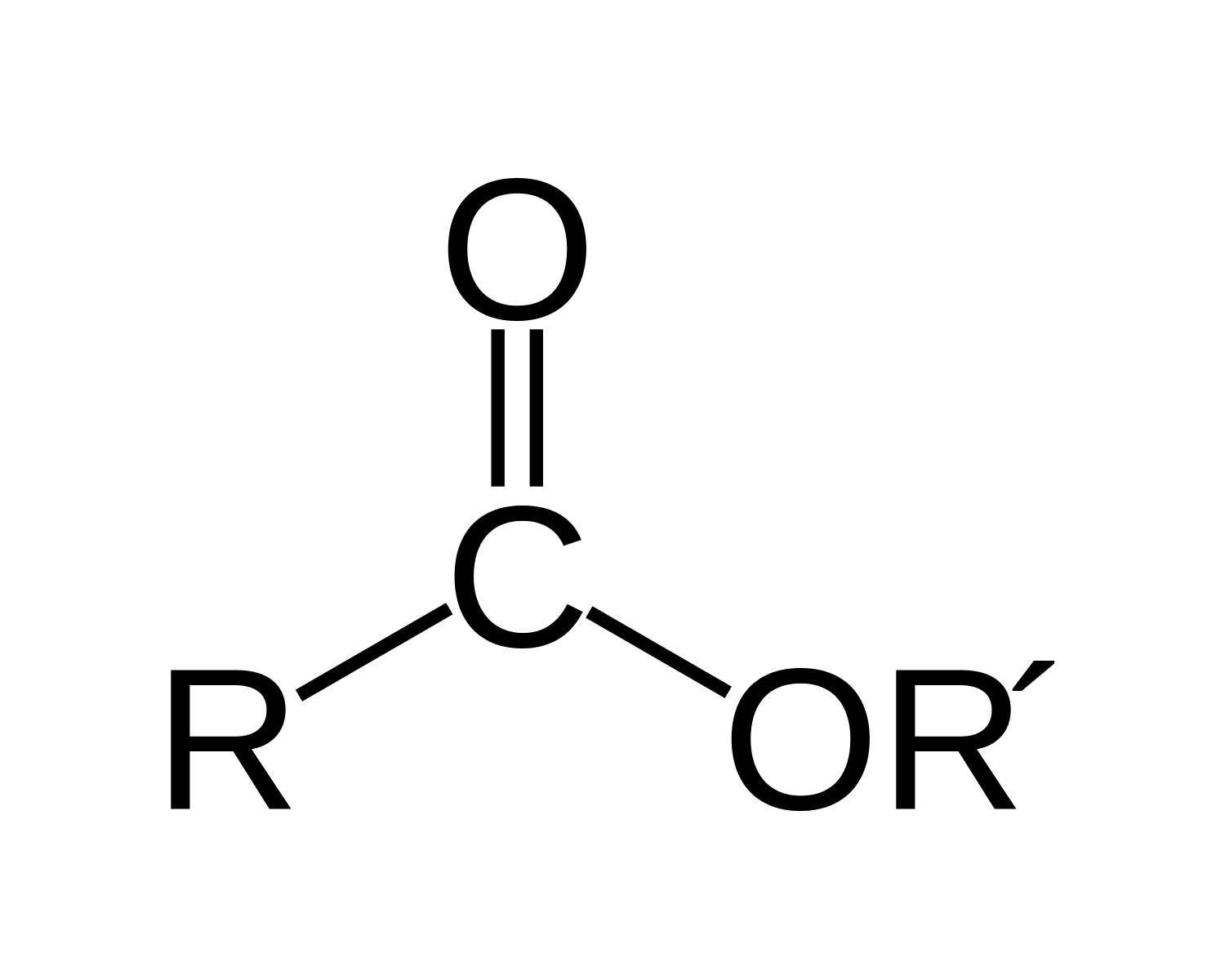
functional group and nomenclature for an ester
•Esters are formed from a condensation reaction between a carboxylic acid and an alcohol, producing a molecule of water. the H is from the alcohol and OH from carboxylic acid.
•prefix is the alcohol
•suffix is the carboxylic acid.
• example-
Ester: CH₃COOCH₂CH₃
Alcohol part: From ethanol (CH₃CH₂OH), which becomes ethyl.
Acid part: From ethanoic acid (CH₃COOH), which becomes ethanoate.
Full Name: Ethyl ethanoate
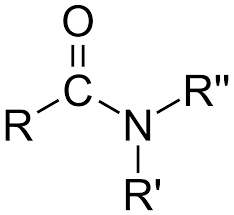
functional group and nomenclature for amides
suffix- amide
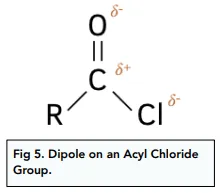
functional group and nomenclature for acyl chlorides
suffix- oyl chloride
•drop the -oic acid from the parent carboxylic acid and replace with -oyl chloride
what is a chiral carbon?
•A chiral carbon is a carbon atom with four different atoms or groups of atoms attached to it.
•creates a tetrahedral structure.
•has optical isomers
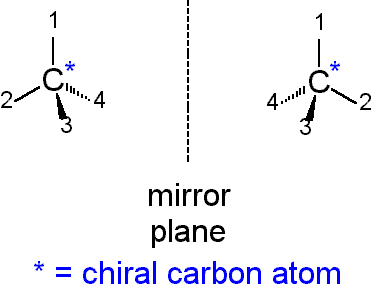
define optical isomer. how can they be distinguished?
•optical isomerism describes stereoisomers that are non-superimposable mirror images of each other, like left and right hands.
•These molecules, called enantiomers, possess the same molecular formula and bonding but differ in their three-dimensional arrangement due to a chiral carbon (a carbon atom bonded to four different groups).
•Optical isomers have the same physical properties but rotate plane-polarized light in opposite directions, a property that distinguishes them.
what is the structural feature of optical isomers that makes them isomers?
they are non super imposable mirror images
define a racemic mixture
a solution containing an equal mixture of enantiomers
how is plane polarised light made? (Diagram)
how does this enable us to distinguish enantiomers?
•when plane polarised light is passed through separate solutions of enantiomers, they rotate the light in equal and opposite directions
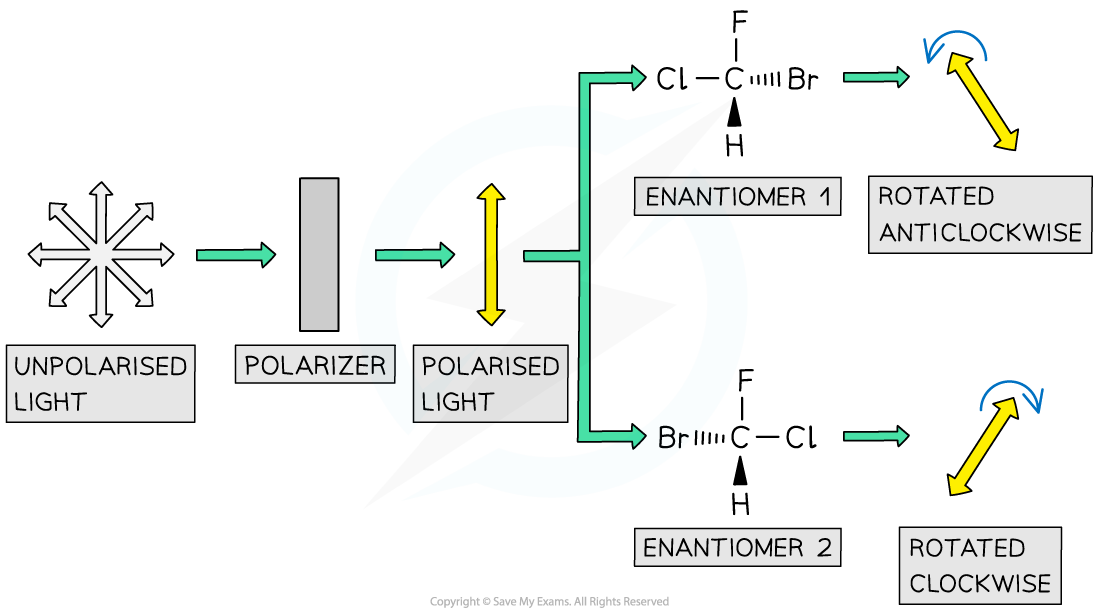
what happens when plane polarised light is passed through a racemic mixture?
when plane polarised light is passed through a racemic mixture of equally mixed enantiomers, they will appear inactive.
this is because the effects of one isomer cancels out the other.
appear optically inactive
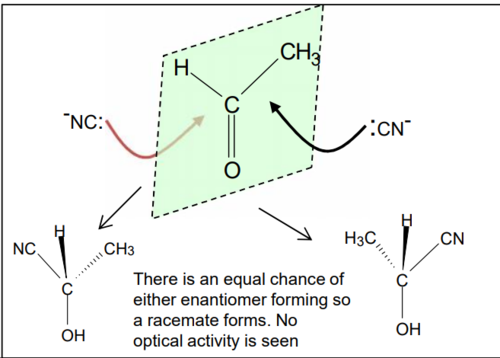
why do we get a racemic mixture with KCN/H2SO4 and NaBH4. explain why the product has no effect on plane polarised light
the planer carbonyl group is equally likely to be attacked above or below its plane
this leads to equal quantity’s of both enantiomers being formed (racemic mixture)
one isomer will rotate plane polarised light clockwise and the other, anticlockwise (equal and opposite directions), so effects will cancel out.
what reagents can distinguish between aldehydes and ketones
•using weak oxidising agents such as…
•Tollens reagent (ammoniacal silver nitrate [(AgNH3)2]+. if aldehyde present a silver mirror precipitate will appear as silver atoms reduced.
•Fehlings solution- Cu2+ oxidised to Cu+. if aldehide present will go from blue to brick red precipitate.
![<p>•using weak oxidising agents such as…</p><p>•Tollens reagent (ammoniacal silver nitrate [(AgNH3)2]+. if aldehyde present a silver mirror precipitate will appear as silver atoms reduced. </p><p>•Fehlings solution- Cu2+ oxidised to Cu+. if aldehide present will go from blue to brick red precipitate. </p>](https://knowt-user-attachments.s3.amazonaws.com/023d7f1c-3943-4da3-8262-8bab143e0950.webp)
what reaction mechanism is used to form hydroxy nitriles
nucleophilic addition
what functional groups form hydroxy nitriles?
aldehydes and ketones
what reagents are used in nucleophilic addition? what is the alternative reagent and why is this not used commercially?
Potassium cyanide/ KCN
Followed by H2SO4
HCN- very toxic
Diagram - reaction mechanism to form hydroxy nitriles
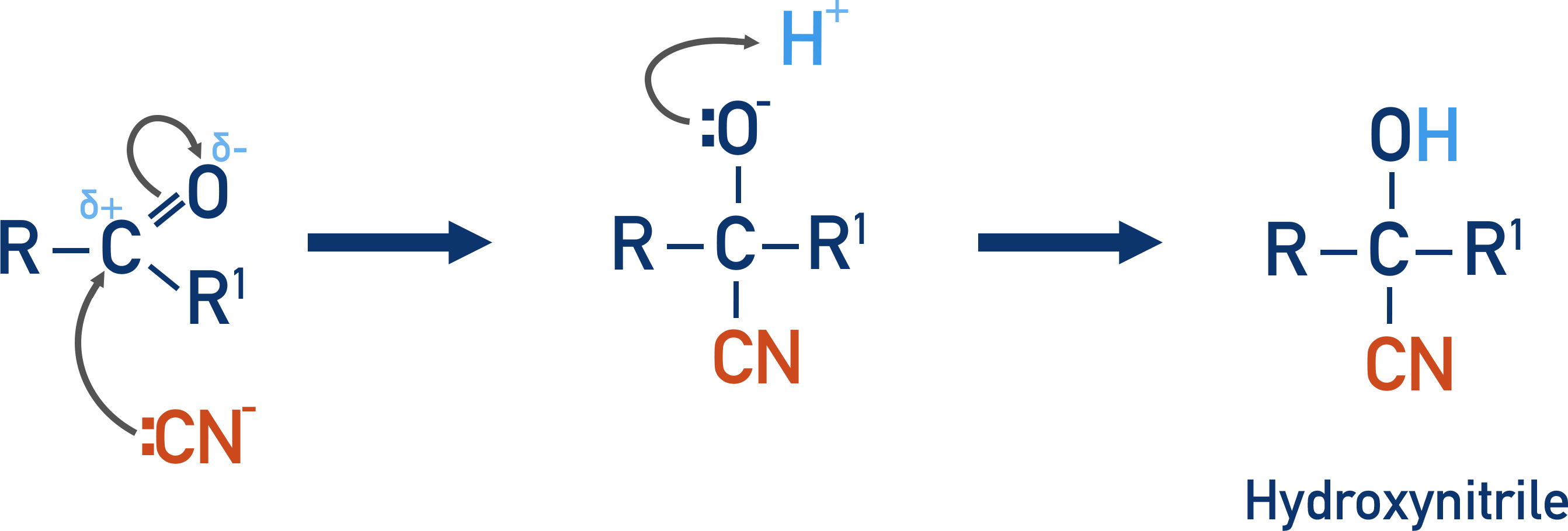
what is the prefix for hydroxy nitriles and why isn’t there a suffix typically
Prefix- Cyano
always highest priority apart from in the presence of a carboxylic acid group
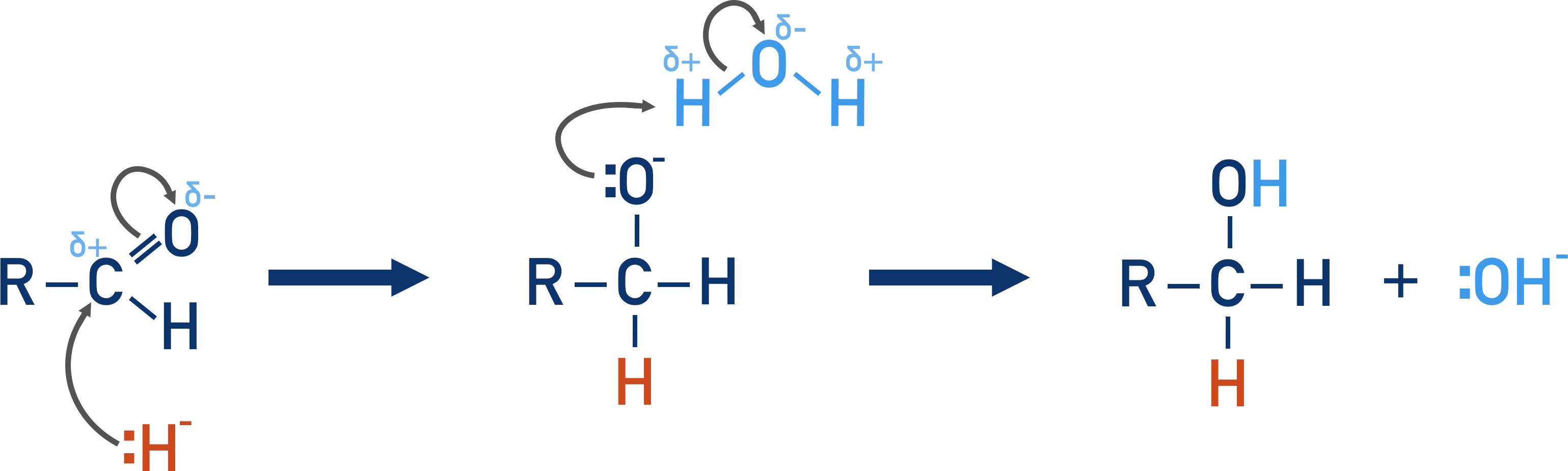
what is the mechanism for the reduction of aldehydes and ketones. state the reagent and products.
nucleophilic addition
sodium borohydride solution (NaBH4)
aldehyde+2[H]—→ Primary alcohol
ketone+2[H]—> secondary alcohol
![<ul><li><p>nucleophilic addition </p></li><li><p>sodium borohydride solution (NaBH4)</p></li><li><p>aldehyde+2[H]—→ Primary alcohol</p></li><li><p>ketone+2[H]—> secondary alcohol </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/4e6edb9c-9552-4ad1-a163-85fa77e4d05d.png)
diagram of the mechanism
ignore the OH on the water and in the products. mechanism only needs H- as nucleophile
what are the two acceptations of an aldehyde and ketone where a racemic mixture isn’t formed?
methanal- no chiral carbon
symmetrical ketones- no chiral carbon
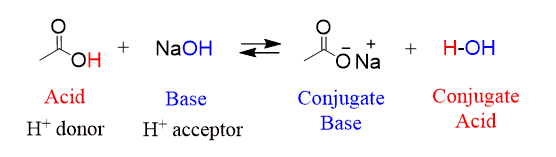
what 3 reactions do acids do?
acid + base—> salt + water
acid + metal —> salt + H2
acid + metal carbonate —> salt + CO2 +H2O (test for carboxylic acid)
why are carboxylic acids not strong acids?
not many H+ ions will dissociate
use reversable reaction arrows
the acid + base reaction of ethanoic acid (CH3COOH) and sodium hydroxide (NaOH)
CH3COOH +NaOH —→ CH3COONa + H2O
the acid + metal carbonate reaction between ethanoic acid (CH3COOH) and sodium hydrogen carbonate (NaHCO3).
Give an observation and use for this reaction.
2CH3COOH + NaHCO3 —>2CH3COONa + H2O + CO2
observation- effervescence
use- to test for carboxylic acid
the acid + metal reaction between ethanoic acid (CH3COOH) and magnesium (Mg)
2CH3COOH + Mg -→ (CH3COO-)2 Mg2+ +H2
Why is these reaction unusual?
unusual to have ionic interactions within a covalent molecule
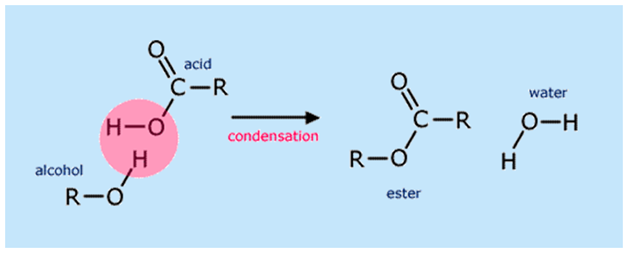
What reaction with carboxylic acid forms an ester? what catalyses this reaction?
carboxylic acid + alcohol —> ester + H2O
concentrated H2SO4
where between the two molecules does an ester bond form?
OH group of carboxylic acid and the H of alcohol
describe briefly how you could measure the melting point of aspirin. how can you tell if its pure?
Method-
put sample in capillary tube
heat rapidly until it gets close to melting point and then heat slowly once close so you don’t miss the point at which it starts melting
record temp that it first starts melting and the temperature at which all the substance is melted
How to tell if its pure-
the closer the melting point to that of pure aspirin, the closer it is to being pure
the smaller the range between start and end melting point, the closer it is to pure aspirin.
impurities weaken intermolecular forces between molecules making melting point lower
what is the general equation for esterification ?
acid + alcohol —c.H2SO4→ ester + water
What are the conditions and catalyst for esterification ?
concentrated H2SO4 reflux
how are esters named
prefix- alcohol
suffix- carboxylic acid
conditions, products and reagents for base hydrolysis of esters
what happens if you further react with HCL?
dilute NaOH
reflux
produces sodium salt of carboxylic acid and alcohol
remove the Na and produce original carboxylic acid
conditions, products and reagents for acid hydrolysis of esters
reflux
H2O + acid catalyst
produces carboxylic acid and alcohol
is acid or base hydrolysis preferred and why ?
base hydrolysis
acid hydrolysis is a reversible reaction so low yield
base hydrolysis is also a faster reaction
Oils and fats (triglycerides) are esters made from …
sodium salts of long chain carboxylic acids
glycerol
how are triglycerides hydrolysed and what are the products? name this reaction
hydrolysed by 3NaOH under reflux
form 3 sodium salts of long chain carboxylic acid
and glycerol
saponification
what are the products of saponification used for?
sodium salts of carboxylic acids used to make salts
glycerol is a good organic solvent- eg acetone
how Is biodiesel formed? name this process.(what do you react with triglycerides and are the products ?)
transesterification
react with 3 molecules of methanol under reflux with KOH catalyst
forms methyl esters of long chain carboxylic acids and glycerol as a waste product
what is biodiesel a mixture of ?
methyl esters
what is the test reagent and observation for acyl chlorides
reagent- H2O
observation- white fumes
what is the mechanism when nucleophiles react with acyl chlorides ?
nucleophilic addition elimination
give the nucleophiles that react with acyl chlorides and products
NH3- amide +HCL
R-OH- ester + HCL
R-NH2- N-substituted amide + HCl
H2O- carboxylic acid + HCl
reaction conditions and observation for nucleophilic addition elimination of acyl chlorides
anhydrous conditions unless using H2O
white fumes, vigorous reaction
disadvantage of acyl chloride reactions
produce toxic HCL gas
what are the industrial advantages and disadvantages of acid anhydrides and acyl chlorides in the manufacture of aspirin?
acyl chlorides-
advantages
very reactive
disadvantages
produces corrosive HCL gas
difficult to handle
acid anhydride-
advantages
cheeper to produce
less corrosive
makes carboxlic acid instead of HCL gas
disadvantages
slower reaction
what 4 nucleophiles react with acid anhydrides during nucleophilic addition elimination? What are the products formed?
H2O- 2x carboxylic acid
R-NH2- N-substituted amide + carboxylic acid
NH3- amide + carboxylic acid
R-OH- carboxylic acid + ester
what is an acid anhydride and how are they named?
formed from condensation reaction of 2 carboxylic acids and produce a molecule of water
suffix- oic anhydride
symmetrical- the name is based on the single parent acid (e.g., ethanoic acid becomes ethanoic anhydride)
asymmetrical- formed from two different acids, the names of both parent acids are listed before the word "anhydride," separated by a space (e.g., ethanoic propanoic anhydride).
define a condensation reaction
the joining of two molecules to form a larger molecule, with the elimination of a smaller molecule (eg water)