Chapt 20
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
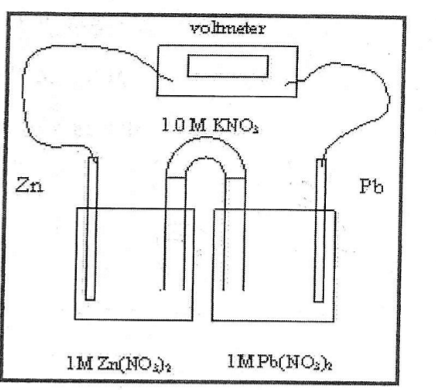
What is Zn in this picture
Anode
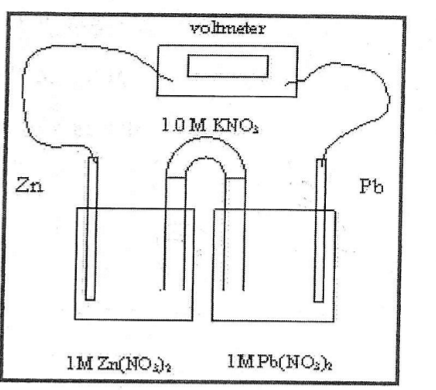
Which way do the K^+ ions migrate
towards the cathode
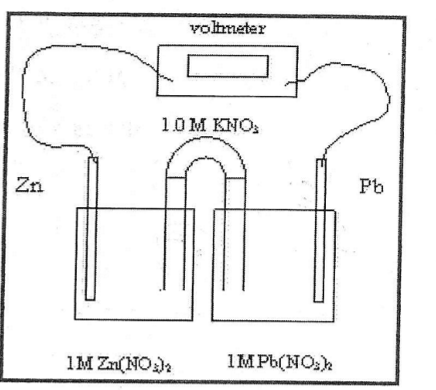
Mass of the the cathode does what in this process
It increases as the electrons flow
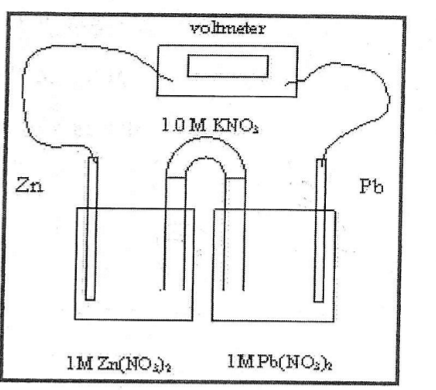
How do the electrons flow
Spontaneously from the anode to the cathode
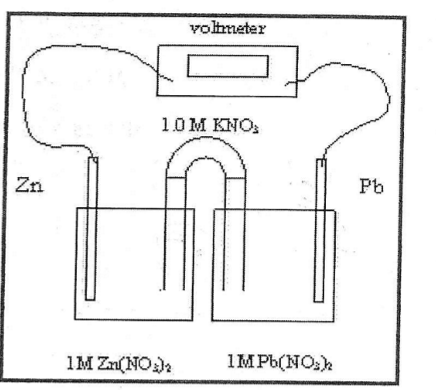
Which is element is the the cathode
Pb
The anode always does what
Oxidizes during the reaction
The cathode always does what
Reduces during the reaction
What comes first in voltaic notation
the anode(meaning oxidation)
What comes after the double line in voltaic notation
the cathode (meaning reduction)
How does a voltaic cell work
A voltaic cell generates electrical energy through spontaneous redox reactions
How does a Electrolytic cell work
generates energy by a non-spontaneous chemical reaction using an external power source, through the process of electrolysis.
The stronger the reduction potential the stronger the what
the oxidizing agent
The strong the oxidizing potential the stronger the what
the reduction agent
The stronger the reduction potential____________ the oxidation potential
the weaker
the stronger the oxidation potential _________ the reduction potential
the weaker