Properties of Water +(Hydrophilic and Hydrophobic Substances)
1/8
Earn XP
Description and Tags
Freshman Mid-term help
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
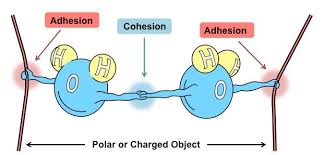
Cohesion
-The attraction between molecules of the same substance.
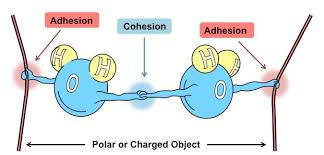
Adhesion
-The type of attraction that happens between two different molecules.
High specific heat
-This helps to regulate cell temperatures in organisms.
Less dense as a solid
-Solid water (ice) is less dense than liquid water 🡪 it floats in liquid water
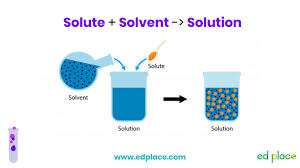
Water Solvent:
Solution
-Uniform mixture of two or more substances
-Ex. Lemonade
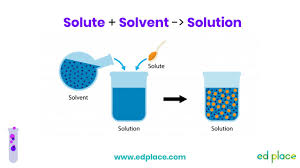
Water Solvent:
Solute
-What gets dissolved
-Ex. Lemonade powder
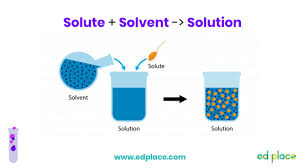
Water Solvent:
Solvent
-Does the dissolving
-Ex. Water
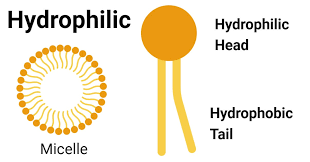
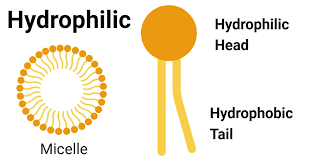
Hydrophilic Substance
- Has an affinity for water
-Water “loving”
-Usually dissolves easily in water
-Ex. salt and sugar
Hydrophobic Substance
-Does not have an affinity for water
-Water “fearing”
-Does NOT dissolve in water
-Ex. oil