Calorimetry & Energy of light
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
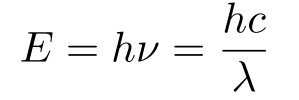
Energy of light
v = frequency in Hz
λ = wavelength in nm (E-9)
h (constant) = 6.626E-34 Js
c (constant) = 3.0E8 m/s
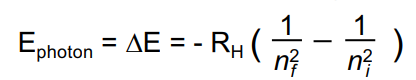
Rydberg’s formula (Only for H atom)
n = energy level
RH (constant) = 2.8E-18
q = msΔT
Calorimetry
q = heat added in J
m = mass in g
s = specific heat in J/g°C
T = temperature in °C
q = -CΔT
Bomb Calorimetry
q = heat added in J
C = heat capacity of calorimeter
ΔT = change in temperature in °C
Endothermic reaction
Heat is absorbed by the reaction
Heat is transferred from the surroundings to the system
Heat can be written as a reactant for the reaction
ΔHrxn is positive
The reactants have lower energy than the products
Exothermic reaction
Heat is given off by the reaction
Heat is transferred from the system to the surroundings
Heat can be written as a product for the reaction
ΔHrxn is negative
The reactants have higher energy than the products
Hess’s Law
The total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps