3.1, 3.2 Kinetic Model of Matter, Gases
0.0(0)
Card Sorting
1/11
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
Define internal energy, U
energy within a substance
2
New cards
Define temperature
measure of avg speed of particles
3
New cards
Define heat
thermal energy transferred from hot subst to colder one
4
New cards
How to convert from C to K?
+273
5
New cards
0K = ?
-273C
6
New cards
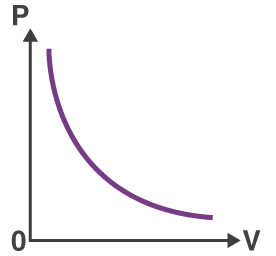
__ Law
At const. t__ & amt of __, P ∝ __ OR P1V1 = __
Boyle’s, temp, gas, 1/V, P2V2
7
New cards
__ Law
At const. p__ & amt of __, V ∝ __ OR V1/T1 = __
Charles’, pressure, gas, T, V2/T2
8
New cards
__ Law
At const. __ & amt of __, P ∝ __ OR P1/T1 = __
Gay Lussac’s, V, gas, T, P2/T2
9
New cards
Combined Gas Law
P1V1/T1 = P2V2/T2
10
New cards
cm3 → m3?
x10^-6
11
New cards
What must be constant for the combined gas law?
# of moles of gas
12
New cards
When melting/boiling, temp is __ & there is i__ of energy
constant, input