Nuclear Chemistry and Radioactivity
1/11
Earn XP
Description and Tags
These flashcards cover key terms and definitions related to nuclear chemistry and radioactivity from the lecture notes.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Significant Figures
All non-zero digits are significant; zeros between non-zero digits are significant; leading zeros are not significant; trailing zeros in decimal numbers are significant.
Atomic Number (Z)
The number of protons in the atomic nucleus, defining the chemical nature of the atom.
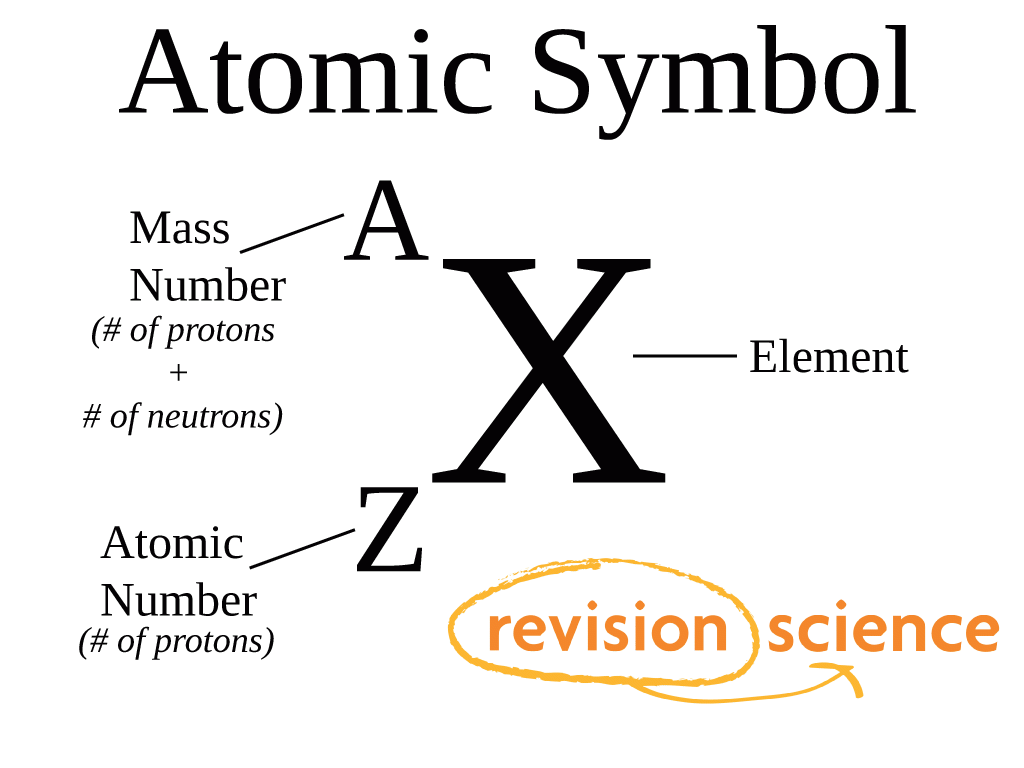
Mass Number (M)
The total number of nucleons (protons and neutrons) in the atomic nucleus.
Nuclide
An atom with a specific mass number and atomic number.
Isotopes
Nuclides with the same atomic number but different mass numbers.
Nucleogenesis
The formation of new nuclei from existing nucleons.
Exothermic Reaction
A reaction that releases energy into the surroundings as heat and radiation.
Half-life
The time required for half of the nuclei in a sample to undergo a decay event.
Decay Constant (k)
A characteristic of a particular radioactive nuclide that does not depend on the amount of sample.
Activity (A)
A measure of the rate of decay, defined as the number of nuclei that disintegrate per second.
Specific Activity
The activity per gram of radioactive nuclide.
Molar Activity
The activity per mole of radioactive nuclide.