Biological Molecules
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
DNA Extraction (Practical)
Steps:
1. Grind sample in mortar & pestle → breaks cell walls.
2. Mix with detergent → breaks membranes, releases cell contents.
3. Add salt → breaks hydrogen bonds between DNA and water.
4. Add protease enzyme → digests proteins associated with DNA.
5. Add alcohol (ethanol/ice cold) → precipitates DNA.
6. DNA appears as white strands → can be spooled onto glass rod.
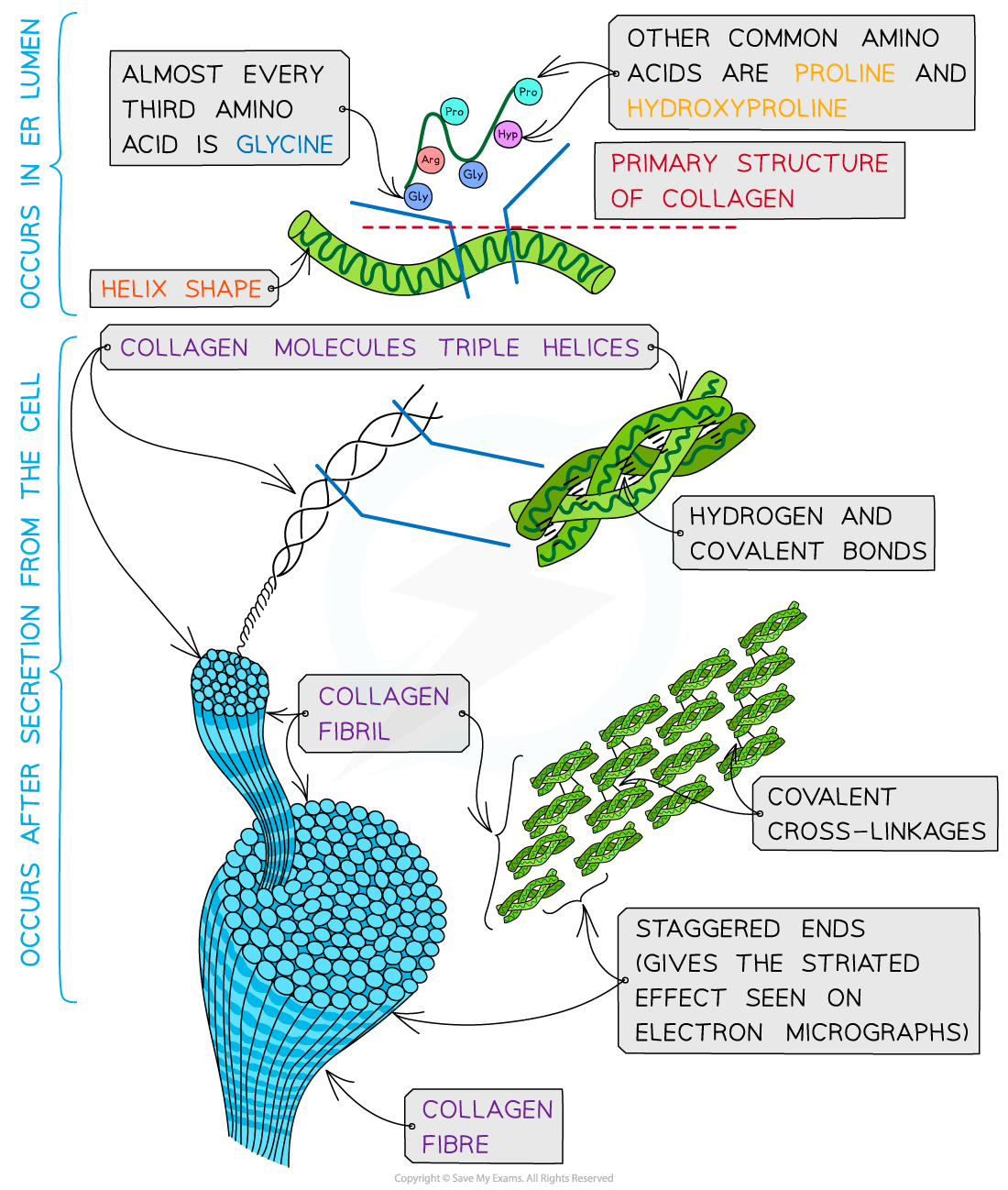
Collagen
Collagen (tendons, ligaments, skin, bones, cartilage):
Each molecule is three polypeptide chains wound into a triple helix.
Glycine every third residue (small side chain) allows tight packing
Hydrogen bonds stabilize the triple helix.
Molecules align staggered and cross-link to form fibrils, which bundle into fibres → very high tensile strength.
Elastin
Elastin (skin, lungs, blood vessel walls):
Made by linking many tropoelastin molecules.
Tropoelastin has alternating hydrophobic regions and lysine-rich areas
Molecules are cross-linked by covalent bonds between lysines, creating an elastic network that stretches and recoils without breaking → elasticity.
Keratin
Keratin (hair, skin, nails): rich in cysteine → many disulfide (S–S) bonds.
More S–S bonds ⇒ harder
Less flexible (nails)
Fewer ⇒ more flexible (hair).
Burning hair/skin smells due to sulfur.
Fibrous Protein
Built from a limited range of amino acids with many hydrophobic R-groups in the primary sequence → repetitive, highly ordered structures
insoluble
mechanical strength rather than metabolic roles.
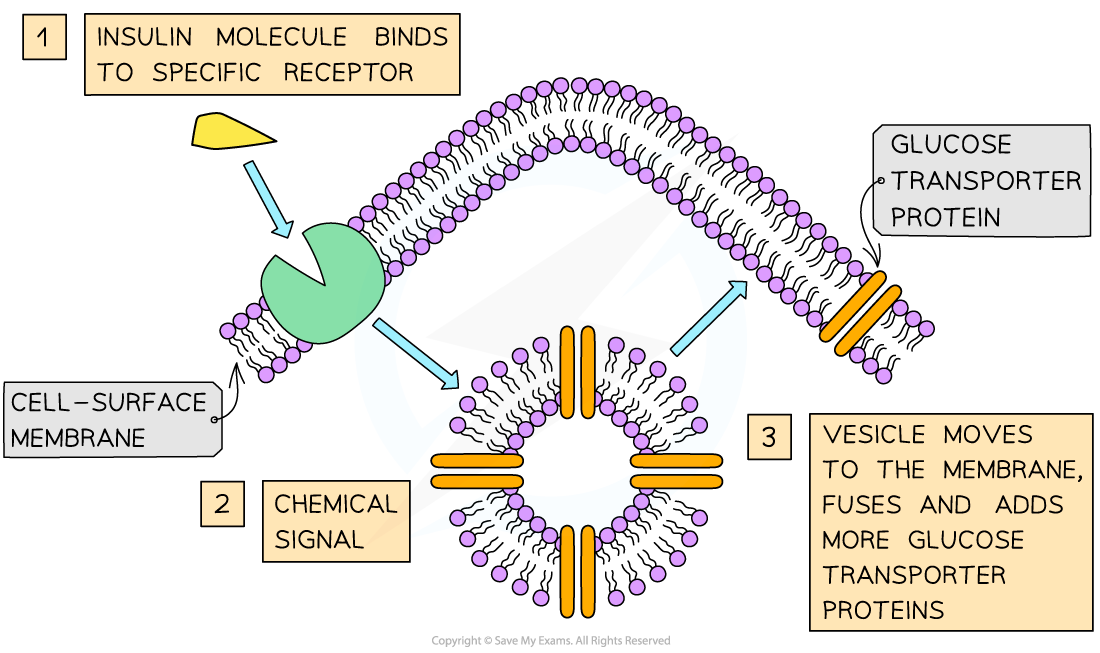
Insulin
Insulin: globular hormone for blood glucose regulation.
Must be soluble for transport in plasma and have a precise shape to fit receptors on cell membranes.
Conjugated Protein: Catalase
Contain a non-protein prosthetic group (may be lipid → lipoprotein, carbohydrate → glycoprotein, metal ions or vitamin-derived groups).
Catalase: enzyme that decomposes H₂O₂ (harmful metabolic by-product). Quaternary protein with haem groups (Fe) that let it interact with hydrogen peroxide and rapidly break it down so it doesn’t damage cells.
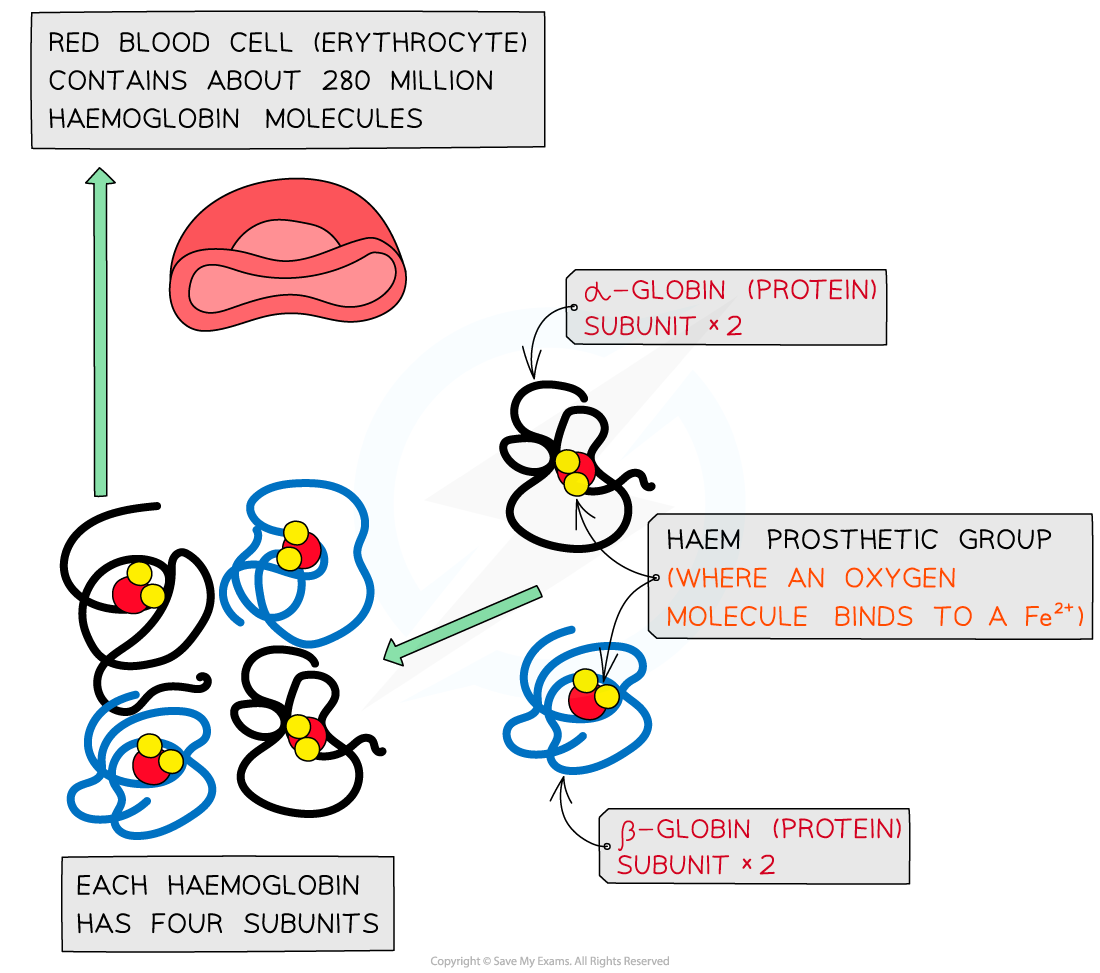
Conjugated Protein: Haemoglobin
Contain a non-protein prosthetic group (may be lipid → lipoprotein, carbohydrate → glycoprotein, metal ions or vitamin-derived groups).
Red oxygen-carrier in RBCs. Quaternary protein of 4 polypeptide subunits (2α + 2β), each with a haem prosthetic group containing Fe²⁺. Iron binds O₂ reversibly, enabling pick-up in lungs and release in tissues.
Globular Proteins
Form when the chain folds so hydrophobic R-groups tuck inside and hydrophilic R-groups face out → soluble in aqueous environments (blood, cytosol).
Vital in regulation and rapid processes: enzymes, hormones, antibodies, transport.
Tertiary Structure
3D folding due to R-group interactions:
Hydrophobic/hydrophilic interactions.
Hydrogen bonds( formed by oppositely charge groups).
Ionic bonds.
Disulfide bonds (covalent, between sulfur-containing R groups).
Cysteine has sulfur. When two cysteines are close together, a covalent bond can form.
Produces complex 3D shape → functional protein.
Order of strength:
Disulfide bonds (covalent) – strongest, permanent cross-links between cysteine residues.
Ionic bonds (salt bridges) – strong electrostatic interactions between oppositely charged R groups.
Hydrogen bonds – weaker than ionic but very numerous, important for shape and stability.
Hydrophobic interactions / van der Waals forces – weakest individually, but collectively contribute a lot.
Primary & Secondary Structure
1. Primary Structure
Sequence of amino acids in polypeptide chain.
Determines final shape and function.
2. Secondary Structure
Forms immediately after being formed in ribosome.
No R Group interactions are present.
Regular structure formed by H-bonds between amino acids (amine & carbonyl groups) causing folding or coiling:
Note: Carbonyl is C=O group
α-helix (coil).
β-pleated sheet (zig-zag sheet).
Thin Layer Chromatography (TLC) – Separating Amino Acids
- Technique for separating/identifying amino acids.
- Components:
- Stationary phase = thin layer of silica gel on sheet.
- Mobile phase = solvent.
- Method:
1. Draw pencil line 2 cm from bottom of plate; mark spots.
2. Add amino acid solution.
3. Place plate in solvent (no more than 1 cm deep).
4. Solvent moves up plate; amino acids separate by solubility + interactions.
5. Remove plate when solvent front ~2 cm from top.
6. Spray with ninhydrin → amino acids visible as purple/brown spots.
7. Measure distance travelled.
- Retention factor (Rf):Rf=distance travelled by solvent/distance travelled by component
- Each amino acid has characteristic Rf value (under identical conditions).
Emulsion Test
1. Mix sample with ethanol.
2. White emulsion = lipid present.
3. Clear = negative.
4. Mix with water + shake.
5. Should form two layers of liquid.
Cholesterol
Manufactured in liver & intestines.
Role:
Positioned between phospholipids.
Adds stability to membranes
cholesterol has hydrophobic and hydrophilic ends that interact with phospholipids (1), acting to make cell membranes more stable/rigid (1)
Prevents membranes becoming too fluid at high temps or too rigid at low temps.
Used to make: Vitamin D, steroid hormones, bile.
Sterols
Known as steroid alcohols.
Not fats/oils, but complex alcohol-based molecules.
Structure: 4 carbon rings + polar –OH group at one end + hydrophobic rest of molecule
Phospholipid behaviour in Water
Form surface films/monolayers (heads in water, tails out).
Form bilayers (hydrophobic tails inside, heads outside).
→ Essential for membranes.
Called surfactants (reduce surface tension) e.g. in lungs
Phospholipids
- Modified triglycerides: contain P, C, H, O.
- One fatty acid replaced by phosphate group (PO₄³⁻).
- Phosphate group = negatively charged → soluble in water.
Structure:
- Hydrophilic head (phosphate group).
- Hydrophobic tails (fatty acid chains).
- → Molecule is amphipathic (dual nature).
Triglycerides
- Made by combining 1 glycerol with 3 fatty acids.
- Glycerol: part of alcohols group (contains –OH).
- Fatty acids: carboxylic acids (–COOH + hydrocarbon chain).
- Reaction: condensation/esterification → forms 3 ester bonds (type of covalent bond) + 3 water molecules.
Functions of Lipids
Protection of vital organs
To prevent evaporation or for waterproofing in plants & animals
To insulate the body
They form the myelin sheath around some neurones
As a water source (water is released as lipids are respired)
As a component of cell membranes
Hormone production
Buoyancy for aquatic animals
Benedict's for Reducing & Non Reducing
Reducing Sugars:
Procedure:
1. Place sample in boiling tube (if solid, grind or blend with water).
2. Add equal volume of Benedict’s reagent (alkaline copper(II) sulfate).
3. Heat gently in a boiling water bath for 5 minutes.
Reaction:
- Reducing sugar donates electrons to Cu²⁺ (blue) → reduced to Cu⁺ (brick-red Cu₂O precipitate).
Non-reducing sugars (e.g. sucrose) do not react with Benedict’s and remain blue (negative result).
To test them:
1. Boil sample with dilute HCl (hydrolyses sucrose → glucose + fructose, both reducing).
2. Neutralise with alkali.
3. Repeat Benedict’s test → positive result if non-reducing sugar was present.
Colorimetry
Benedict’s test colour intensity depends on sugar concentration. A colorimeter measures absorbance or transmission of light by the solution. More concentrated solution = more light absorbed, less transmitted.
Procedure:
Filter sample.
Calibrate colorimeter with distilled water.
Perform Benedict’s test on range of glucose concentrations.
Filter solutions to remove precipitate.
Measure % transmission of each solution with colorimeter.
Plot calibration curve of % transmission vs concentration.
Use curve to find concentration of unknown solution.
Starch & Glycogen Location
Starch- grains in storage organs (tubers or seeds or in chloroplast)
Glycogen- granules stored in the cytoplasm of liver & muscle cells.
Cellulose Fibres
Cellulose chains form hydrogen bonds with each other → microfibrils.
Microfibrils bundle into macrofibrils, which form fibres
Fibres = strong, insoluble, used to make cell walls.
Cellulose = dietary fibre/roughage:
- Hard to digest (humans lack cellulase enzyme).
- Provides fibre essential for healthy digestive system.
- High tensile strength
Cellulose
Made from β-glucose (not α-glucose).
β-glucose molecules cannot join unless alternate molecules are rotated 180°.
This allows 1–4 glycosidic bonds to form.
Results in a straight, unbranched chain
Key shared properties of amylopectin & glycogen:
Insoluble.
Branched.
Compact.
Adapted to efficient storage and rapid glucose release.
Glycogen
Main energy storage polysaccharide in animals and fungi (functionally equivalent to starch in plants).
Highly branched due to more alpha 1-6 bonds than amylopectin.
Compact, requires less storage space, more insoluble structure.
Branching = many free ends → glucose can be rapidly added/removed.
Important for mobility in animals, ensuring a constant energy supply.
Amylopectin
1–4 glycosidic bonds between α-glucose molecules.
–6 glycosidic bonds, creating branching points.
Branches occur ~ every 25 glucose subunits.
Branched structure = more compact & less soluble than glucose
Efficient storage.
Amylose
Long chain of α-glucose monomers
1–4 glycosidic bonds.
Helix, (hydrogen bonding)
Helical structure -> compact & less soluble than glucose.
Efficient for storage
Monosaccharides of the following:
Maltose
Sucrose
Lactose
Glucose & Glucose
Fructose & Glucose
Galactose & Glucose
Water Properties Essential for Life
1. Solvent:
- Polar molecules (e.g., amino acids, nucleic acids, salts) dissolve in water.
- Important for cytosol of prokaryotes and eukaryotes, and for transport of ions/molecules.
- Salt= ionic= polar = can interact with water (dissolve)= hydrophilic (water loving)
- BUT Oil= non polar= does not dissolve= hydrophobic (or polysaccharides)
2. Transport medium:
- Cohesion allows water to move as one mass.
- Capillary action = adhesion + cohesion → enables water to rise in narrow tubes (important in plants).
3. Temperature stabiliser:
- Acts as a coolant.
- Absorbs large amounts of energy to break hydrogen bonds → buffers organisms from rapid temperature changes.
- Helps maintain stable enzyme function within the narrow temperature range required.
4. Habitat:
- Aquatic organisms depend on stable water environments.
- Ice floats → insulates water beneath, preventing freezing solid.
- Surface tension allows some organisms to live on water (e.g., pond skaters).
Characteristics of Water
Unusually high boiling point for a small molecule:
- Caused by hydrogen bonding, which requires lots of energy to break.
- Explains why water is liquid at room temperature, unlike CO₂ or O₂.
Freezing behaviour:
- Water expands when frozen.
- At 4°C and below, hydrogen bonds fix molecules in a rigid, open, tetrahedral lattice → ice less dense than liquid water → floats.
Cohesion & Adhesion:
- Cohesion: molecules stick to each other → enables water transport in plants (e.g., drawn up xylem).
- Adhesion: molecules attracted to other surfaces → e.g., water wets skin but doesn’t run off.
Surface tension:
- Cohesion creates a ‘skin’ at the surface.
- Strong enough to support small organisms (e.g., pond skaters).