The photoelectric effect
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is the photoelectric effect?
When light of a particular frequency is incident on a metal surface causing electrons to be emitted from the surface.
What does the photoelectric effect show?
-higher intensity of light doesn't change the kinetic energy of the electrons
-1 photon is absorbed by 1 electron
-provides evidence for the particle nature of light
How does the photoelectric effect show light behaves as a quanta?
If light behaved as a wave, light of any frequency would eventually deliver enough energy to the electrons to liberate them.
What is the equation for energy of a photon?
E=hf
What is the threshold frequency?
The lowest frequency of light that causes electrons to be emitted.
What is the work function?
The minimum photon energy needed to release an electron from the surface of the metal.
What does the experiment for the photoelectric effect look like?
Shine light on plate, electrons emitted and jump to opposite metal plate. Battery set up with negative terminal facing incoming electrons so will try to stop electrons from reaching other plate. To measure Ke of electrons, turn voltage up on battery until no electrons reach the other plate. This voltage is the stopping potential (Vs). For any voltage V=E/Q. E should match Ek of electrons coming off plate, V matches Vs, and Q matches charge of electron (e) (1.6*10^-19) so Vs=Ek/e.
Why does Vs=Ek/e become Ek max= eVs
Ek becomes Ek max since we are only intrested in the elctrons liberated from the surface so they will have the max Ek.
What us the graph of Ek max against frequency for the photoelectric effect?
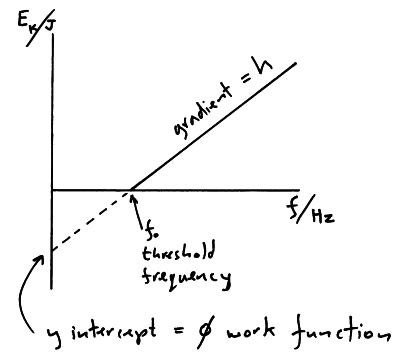
How do you get the equation Ek max=hf -∅
Look at the straight line from the photoelectric effect graph. y=mx+c becomes Ek max = hf- ∅
energy left over for an electron after it's been liberated= energy given to electron by photon - the energy needed to liberate the electron.
What happens in the photoelectric effect when the frequency is equal to the threshold frequency?
electrons will have enough energy to be liberated but no energy left over to be used as Ek. In this case 0=hf - ∅. Where 0 = Ek and f= threshold frequency. therefore f (threshold frequency) = ∅/h
How do different metals affect the photoelectric effect?
Different metals have different threshold frequencies and work functions.
In the photoelectric effect what does increasing the intensity of radiation of the plate do?
increases the number of photon incident on the metal plate hence the number of photoelectrons.
What is the photoelectric current?
A measure of the number of photoelectrons emitted per second. It is calculated by the number of electrons emitted multiplied by the charge of one electron. It's proportional to the intensity of incident radiation.