Paper 3
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
Ideal Gas Equation
pV = nRT
p = Pascals
V = m3
R = 8.314
T = K (+273C)
Polar/Non polar molecules
Dipoles across covalent bonds are caused by significant differences in electronegativity values of bonded atoms (>0.4)
C-H bonds classed as non-polar
Why aren’t symmetrical molecules polar?
Symmetrical molecules are generally non polar. Polar bonds are arranged in a way that the individual bond dipoles cancel each other out, leaving no overall dipole on the molecule
Why are non-symmetrical molecules polar?
Individual bond dipoles do not cancel out.
What causes asymmetry?
Asymmetry caused by presence of different terminal atoms, or presence of lone pairs around central atom makes molecules non symmetrical
Electron structure
Orbitals can only hold 2 electrons
s orbitals = spherical shape
p orbitals = dumbbell shape
s subshells can hold 2 electrons
p subshells can hold 6 electrons
d subshells = 10 electrons
f subshells = 14 electrons
Draw an enthalpy cycle, or otherwise, to show how ΔrH may be determined using average bond enthalpies.
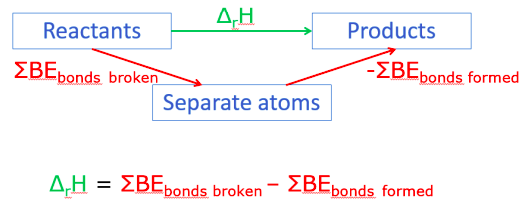
Enthalpy changes
△H = IN - OUT
EIN = Energy needed to break the bonds between atoms in the reactants
EOUT = Energy released when bonds between atoms formed in the products
Calorimetry questions
q = mc△T
The m is the mass of liquid the thermometer is in
Don’t forget to multiply △H values by the number of moles in the equation
△H values must have a sign ±
Why are the experimental calorimetry results different to the data book values?
heat loss to surroundings
non standard conditions
incomplete combustion (alcohols)
specific heat capacity of apparatus ignored
How to calculate Ecell?
Epos - Eneg
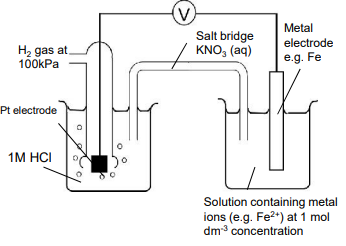
Draw a diagram to measure Eo value.
Draw with SHE
Salt bridge allows a flow of ions
Fuel cells
reactants supplied constantly
Storage cells (batteries)
reactants stored in the cell
Kc or Kp > 1
Equilibrium position is to the right/products side
Kc or Kp >> 1
Equilibrium position is REALLY to the right/products side
Kc or Kp < 1
Equilibrium position is to the left/reactants side
Kc or Kp << 1
Equilibrium position is REALLY to the left/reactants side
Partial pressures should add up to
total pressure
Mole fractions should add up to
1
The value of Kc and Kp only alters by changes in what?
Temperature
Transition element definition
Forms at least 1 ion with an incomplete d subshell
What 2 examples are not transition elements?
Sc and Zn
Sc only forms Sc3+ (which is 3d0)
Zn only forms Zn2+ (which is 3d10)
What’s unusual with Cu and Cr?
Cu and Cr are unusual with their 4s1 configuration rather than the normal 4s2
Hydroxide ppt
All aqueous TM ions produce hydroxide ppt with a small amount of OH-
Only Cr(OH)3 dissolves in excess to form [Cr(OH)6]3-
All aqueous TM ions produce hydroxide ppt with small amount of NH3
Only Cu(OH)2 and Cr(OH)2 dissolve in excess to form [Cu(NH3)4(H2O)2]2+ and [Cr(NH3)6]3+
![<ul><li><p>All aqueous TM ions produce hydroxide ppt with a small amount of OH<sup>-</sup></p><ul><li><p>Only Cr(OH)<sub>3</sub> dissolves in excess to form [Cr(OH)<sub>6</sub>]<sup>3-</sup></p></li></ul></li><li><p>All aqueous TM ions produce hydroxide ppt with small amount of NH<sub>3</sub></p><ul><li><p>Only Cu(OH)<sub>2</sub> and Cr(OH)<sub>2</sub> dissolve in excess to form [Cu(NH<sub>3</sub>)<sub>4</sub>(H<sub>2</sub>O)<sub>2</sub>]<sup>2+</sup> and [Cr(NH<sub>3</sub>)<sub>6</sub>]<sup>3+</sup></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/60ec56ac-fa3b-4b79-bd59-a773da350845.png)
Isomerism in complexes
3x bidentate ligand complexes show optical isomerism
2x monodentate / 2x bidentate ligands show trans isomerism, and do not show optical isomerism

Test for NH4+
Add NaOH (aq) and warm gently
Test NH3 gas with damp red litmus paper.
Damp red litmus paper turns blue
NH4+ + OH- → NH3 + H2O

Test for Fe2+
Add NaOH (aq) until in excess
Pale green ppt, insoluble in excess NaOH
Fe2+(aq) + 2OH- (aq) → Fe(OH)2 (aq)

Test for Fe3+
Add NaOH (aq) until in excess
Orange/brown ppt, insoluble in excess NaOH
Fe3+(aq) + 3OH- (aq) → Fe(OH)3 (aq)

Test for Cu2+
Add NaOH (aq) until in excess
Pale blue ppt, insoluble in excess NaOH
Cu2+(aq) + 2OH- (aq) → Cu(OH)2 (aq)

Test for Mn2+
Add NaOH (aq) until in excess
Pale brown ppt, insoluble in excess NaOH
Mn2+(aq) + 2OH- (aq) → Mn(OH)2 (aq)

Test for Cr3+
Add NaOH (aq) until in excess
Grey-green ppt, soluble in excess NaOH, forming green solution
Cr3+(aq) + 3OH- (aq) → Cr(OH)3 (aq)
Cr(OH)3 (aq) + 3OH- → [Cr(OH)3]3+
Test for Carbonate
Add dilute HNO3 (aq) and test gas by bubbling through limewater
Bubbles/effervescence
Limewater turns cloudy
CO32-(aq) + 2H+(aq) → CO2 + H2O
Test for Sulfate
Add Ba2+ (like Ba(NO3)2)
White ppt
Ba2+ + SO42- → BaSO4
Test for Halides
Add AgNO3 followed by NH3
White ppt of AgCl, soluble in dilute NH3
Cream ppt of AgBr, soluble in conc NH3
Yellow ppt of AgI, insoluble in conc NH3
Ag+ + Cl- → AgCl
Ag+ + Br- → AgBr
Ag+ + I- → AgI
Correct order for anion tests
Carbonate
Sulfate
Halide
Why are alkanes unreactive?
High bond enthalpies of C-C and C-H bonds
C-C and C-H bonds are non polar
Why are alkanes bad at making a single organic substance?
Multiple termination steps
Further substitution
Substitution can be anywhere on the C chain
Oxidation of secondary alcohols
Only makes a ketone
Oxidation of tertiary alcohols
Doesn’t oxidise
Amines
Can be primary, secondary, or tertiary
Acts as bases (can accept H+ using lone pair on N → Ammonium salts
Primary aliphatic amines made from haloalkanes with excess ammonia dissolved in ethanol
Haloalkane → Primary aliphatic amines
Excess ammonia dissolved in ethanol
Excess is used to avoid further substitution to secondary and tertiary amines
Nitrobenzene → Aromatic Amines
Sn and conc HCl
6 moles of [H] per NO2 group reduced
Nitriles → Amines
H2 / Ni catalyst
(CN → CH2NH2, so 2H2 is needed)
Primary, secondary, tertiary amides
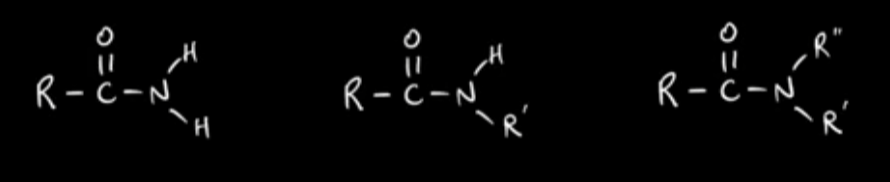
How is the amide group hydrolysed?
With hot aqueous acid and alkali
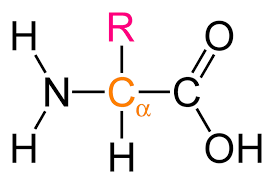
a-amino acids
Carboxylic acid + metal
Carboxylic acid + metal oxides, metal hydroxides
Carboxylic acid + carbonates
Carboxylic acid + metal → Carboxylate + Hydrogen
Carboxylic acid + metal oxides, metal hydroxides → Carboxylate salt + water
Carboxylic acid + carbonates → Carboxylate salt + water + carbon dioxide
Carboxylate salts
(RCOO-)nMn+
(RCOO-)2M2+
NH2 → Ammonium salts
React with acids
There are 2 ways to form Nitriles. What are they?
From haloalkanes - Nucleophilic Substitution
Haloalkane → Nitriles
From aldehydes & ketones - Nucleophilic Addition
Carbonyl → Hydroxynitrile
Carbonyl → Hydroxynitrile
NaCN
Dilute H2SO4 (or H+)
RTP
Nucleophilic addition
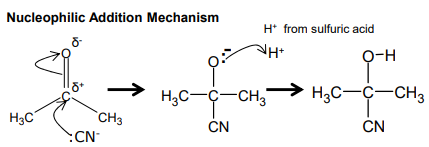
Haloalkane → Nitriles
NaCN dissolved in ethanol
Heat under reflux
Nucleophilic Substitution
This reaction increases the chain length. In this reaction, 1-bromopropane forms butanenitrile
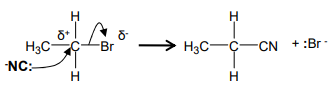
Nitrile → Carboxylic acid
H+ / H2O (could be HCl(aq))
Heat under reflux
Optical Isomerism
Same structural formula, different spatial arrangement of atoms/groups
Non superimposable mirror images of each other (enantiomers)
Possible if a molecule has a chiral centre
For each chiral centre → Pair of optical isomers (Total number of isomers= 2no. of chiral centres)
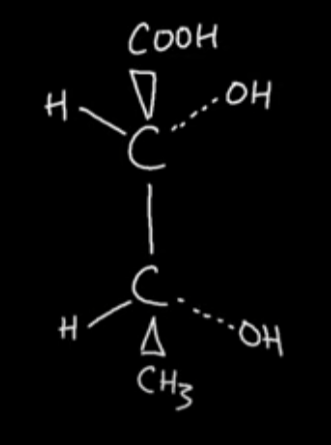
Draw the optical isomer of this molecule with 2 chiral centres
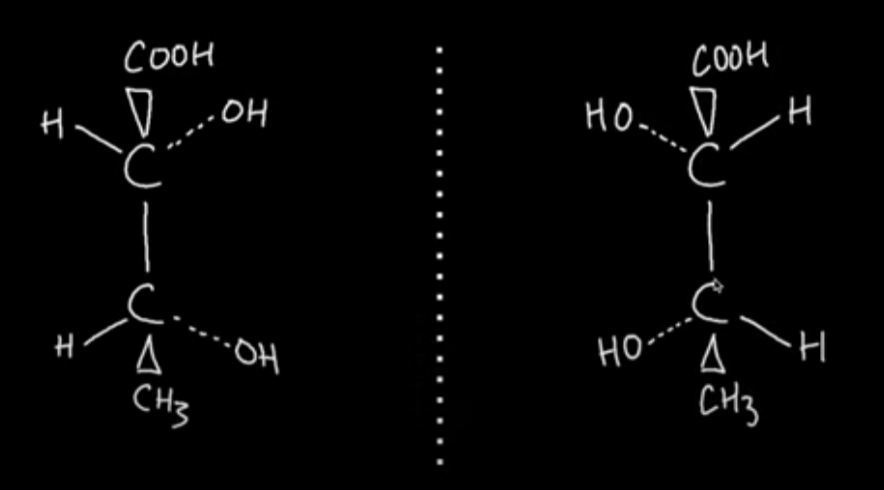
Carbon-Carbon bond formation
Haloalkane → Amine
Need to generate the nitrile (-CN) functional group by reaction with ethanolic CN- then react nitrile with H2/Ni to generate the amine
Haloalkane → Carboxylic acid
Need to generate the nitrile (-CN) functional group by reaction wit ethanolic CN- then react nitrile with HCl to generate the carboxylic acid
C-C bonds also formed when carbonyls → hydroxynitriles
C-C bonds formed in alkylation and acylation reactions with benzene rings
Synthesis of an Organic Solid
Dissolve the impure solid crystals in a minimum amount of hot solvent
Leave to cool and allow crystals to reform
Filter the crystals under reduced pressure
Wash with a little cold solvent
Allow purified crystals to dry
Buchner Flask Diagram
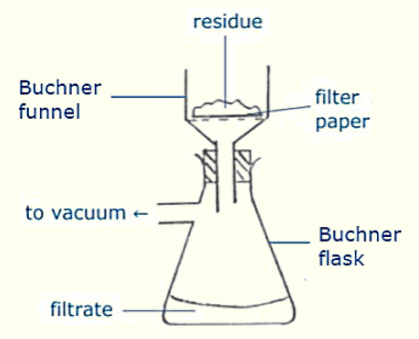
How to determine purity of an organic solid?
Measure melting point and comparing to known data values
Run TLC and measure Rf value to compare to known data values
Run TLC and compare to chromatogram of a pure sample
Run NMR/IR/Mass spectrum, to compare spectral database of pure compound
Distillation Apparatus Diagram
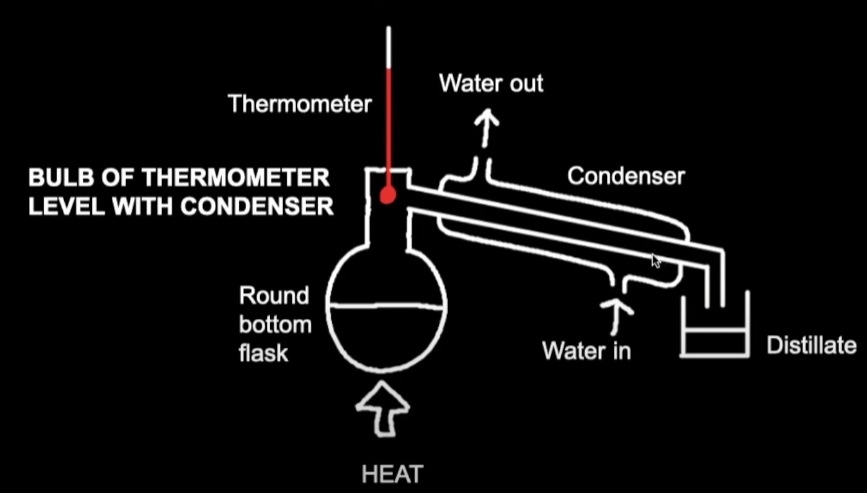
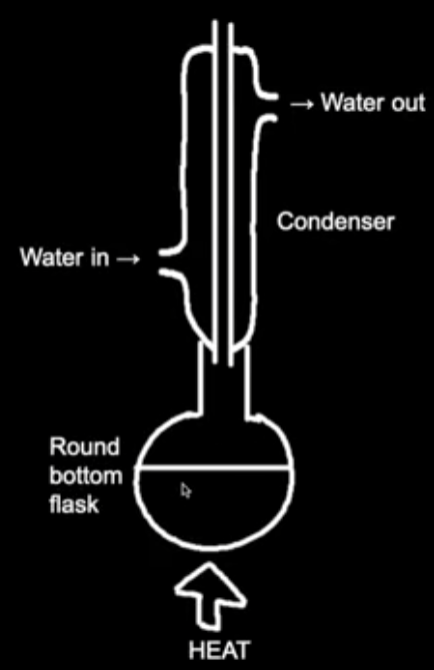
Heat under reflux Apparatus Diagram
Tests for Organic Functional Groups
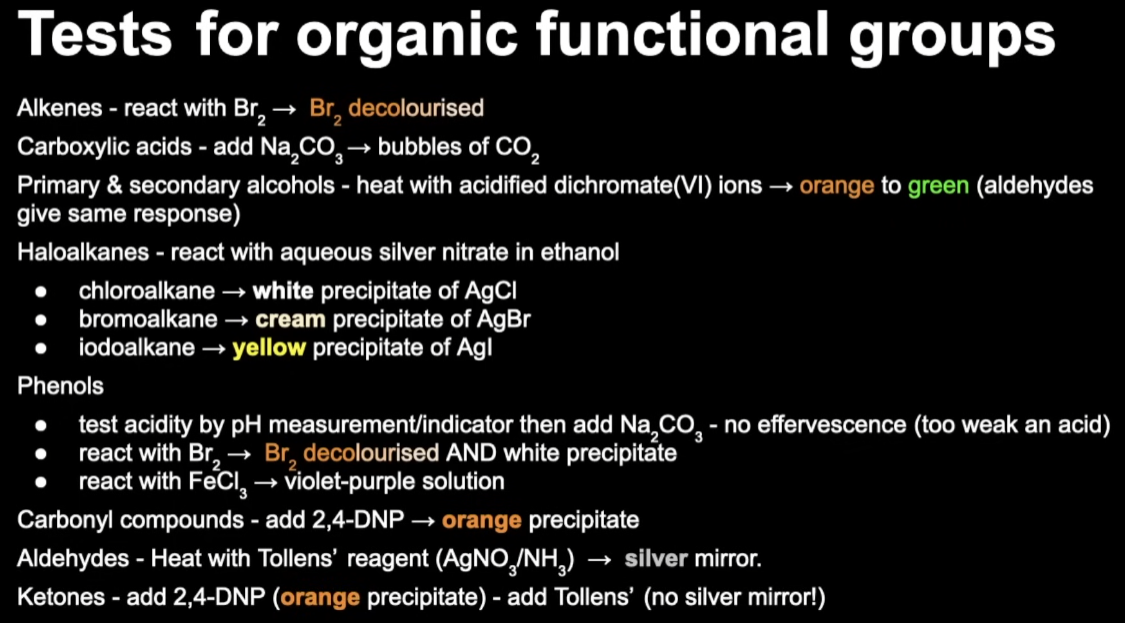
Thin Layer Chromatography
Separation by relative adsorption - components in the mixture bind to the solid stationary phase by differing amounts (strongly adsorbed not moved as far as weakly adsorbed)
How do you find out the substances in TLC?
Calculate Rf value and compare Rf value with known data values
Gas Chromatography
Separation by relative adsorption if stationary phase is solid
Separation by relative solubility if stationary phase is liquid
Retention times (time from injection to detection)
How do you know what you got in gas chromatography?
Compare retention times to known data values
What does the area under peaks in gas chromatography represent?
Relative amounts of components in sample.
The relative proportions are measured by dividing area under peak by the total area under all the peaks.
If you want to know the actual concentrations of the substance in gas chromatography, what would you do?
Measure the area under peak for known concentrations
Then plot a calibration curve.
Concentration is found by comparing the area under a sample peak to calibration curve
How to reduce carbonyls?
NaBH4
Catalytic Hydrogenation (H2/Ni catalyst)
Carboxylic acid → Acyl chloride
SOCl2
Acyl chloride
SO2
HCl
Acyl Chloride → Carboxylic acid
Water
Acyl chloride → Ester
Alcohol
How would the the enthalpy changes of hydration of F- and Cl- differ?
∆hyd of F- would be more exothermic than ∆hyd of Cl- because F- has a smaller size than Cl-
What is the equation and how does entropy change in the standard enthalpy change of atomisation of iodine?
I2 (s) → I2 (g)
The entropy increases because there is a state change from solid to gas, and gas has more disorder
The lattice enthalpy of sodium oxide is more exothermic than that of potassium oxide. Explain why.
The ionic radius of Na+ is smaller.
Na+ has a stronger attraction to O2-
Why is it difficult to predict whether the enthalpy change of solution becomes more exothermic or less exothermic down the group from MgF2 to MgI2
Halide ion gets larger down the group
Lattice enthalpy is less exothermic down group
Hydration enthalpy less exothermic down group
Difficult to predict whether lattice enthalpy or hydration enthalpy has a bigger effect
Does hydration enthalpy increase or decrease down group?
Decrease
Enthalpy change of hydration
The enthalpy change when one mole of gaseous ions is dissolved in water to give one mole of aqueous ions
Enthalpy change of solution
The enthalpy change when 1 mole of a substance dissolves
Standard Enthalpy Change of Reaction
The enthalpy change when the reactants in the stoichiometric equation react to give the products under standard conditions
Standard Enthalpy Change of Formation
The enthalpy change when one mole of a compound is formed from its elements under standard conditions.
Standard Enthalpy Change of Combustion
The enthalpy change when one mole of a substance reacts completely with oxygen under standard conditions
Lattice Enthalpy
The energy change when one mole of an ionic compound is formed from its gaseous ions under standard conditions.
Enthalpy change of Atomisation
The enthalpy change when one mole of gaseous atoms are formed from the element in its standard state.
First electron affinity
The enthalpy change that takes place when one electron is added to each atom in one mole of gaseous atoms to form one mole of gaseous 1- ions.
Average bond enthalpy
The breaking of one mole of bonds in gaseous molecules
Weak Acid Equations

Strong Bases equations
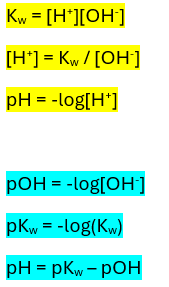
Buffer Equation
[H+] = Ka x ([HA]/[A-])

“Strong acid – Strong base” pH curve
pH = 1
pH = 13
pH = 7
Volume at neutralisation = 25cm3
Steep between pH 4-10
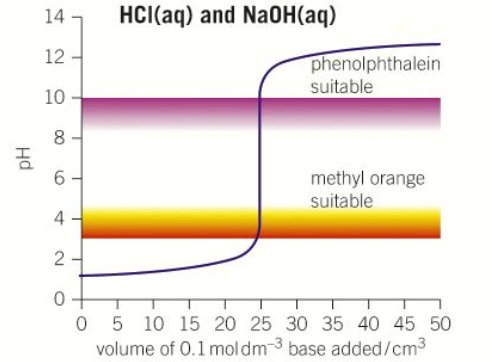

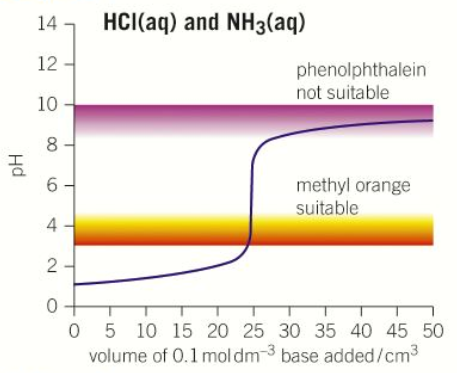
“Strong acid - Weak base” pH curve
pH = 1
pH = 9
pH = Less than 7
Volume at neutralisation = 25cm3
Steep between pH 4-7

“Weak acid – Strong base” pH curve
pH = 3
pH = 13
pH = More than 7
Half neutralisation volume x2
Steep between pH 7-10
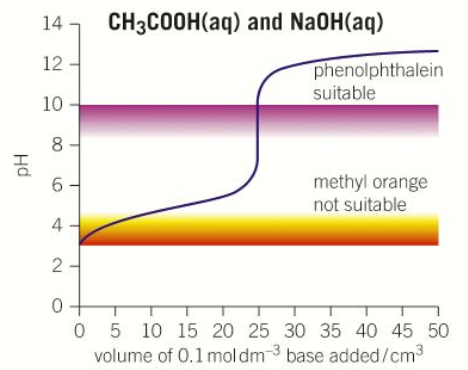

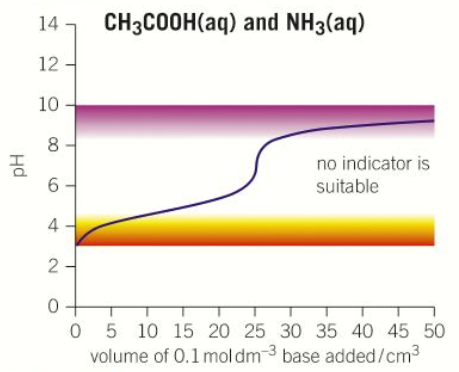
“Weak acid - Weak base” pH curve
pH = 3
pH = 9
No equivalence point
No volume at which equivalence occurs
No steep part of curve
Why is CO toxic to humans?
O₂ co-ordinately bonds to Fe²⁺
When required, O2 is replaced by H2O or CO2
CO forms stronger bonds than O2 so prevents O₂ from attaching to haemoglobin. With CO, its stability constant is greater than with complex in O₂
Proton Exchange
Since D2O has an even number of nucleons (2H) its nucleus does not possess spin property, so do not give a signal in 1HNMR
Definition of E/Z isomer
There is restricted rotation around the C=C double bond. There are two different groups/atoms attached both ends of the double bond.
Definition of Cis/Trans Isomers
A type of E/Z isomerism in which two of the substituent groups are the same.
What’s a zwitterion?
A zwitterion is a molecule that has both a positive and a negative charge - but the overall charge is neutral.