Thermodynamics and Gibbs Free Energy Concepts
1/48
Earn XP
Description and Tags
Created with AI, gone back through and added some practice problems, check the 2nd knowt for the rest of the practice problems
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
Spontaneous Process
A process that can proceed without any outside intervention.
What is the relationship between ΔG and the equilibrium constant (K)?
The relationship can be expressed with the equation: ΔG° = -RT ln(K), where ΔG° is the standard Gibbs free energy change, R is the gas constant, T is the temperature in Kelvin, and K is the equilibrium constant. If K > 1, ΔG° is negative, indicating a spontaneous process and favoring products. If K < 1, ΔG° is positive, indicating a nonspontaneous process and favoring reactants.
Breakdown:
• ΔG = Gibbs free energy change
• R = gas constant (8.314 J/mol·K)
• T = temperature in Kelvin
• K = equilibrium constant
• ln(K) = natural log of K
Important Notes:
• If K > 1, then ln(K) > 0, so ΔG < 0 → reaction is spontaneous.
• If K < 1, then ln(K) < 0, so ΔG > 0 → reaction is non-spontaneous.
• If K = 1, then ΔG = 0 → system is at equilibrium.
Let me know if you need this rearranged to solve for K or used in a calculation.
∆E and ∆H are __ functions
state functions
Enthalpy change
Enthalpy change (ΔH) is the heat absorbed or released by a system at constant pressure during a chemical reaction.
Nonspontaneous Process
A process that requires energy input to occur.
First Law of Thermodynamics
Energy cannot be created or destroyed, only transferred. Total energy change of the universe (ΔE_universe) = 0.
What is the relationship between internal energy (E), work (W), heat (q), and enthalpy (H) in thermochemistry?
The relationship can be expressed with the equation: ΔE = q + W. Additionally, for processes at constant pressure, ΔH = ΔE + PΔV, where ΔH is the change in enthalpy.
Enthalpy (ΔH)
The measurement of heat content in a system; often expressed in kJ/mol.
Exothermic Reaction
A reaction that releases energy, characterized by a negative ΔH.
•Given the following equations and ∆Ho values, determine the heat of reaction at 298 K for the reaction:C2H4(g) + 6 F2(g) →2 CF4(g) + 4 HF(g)
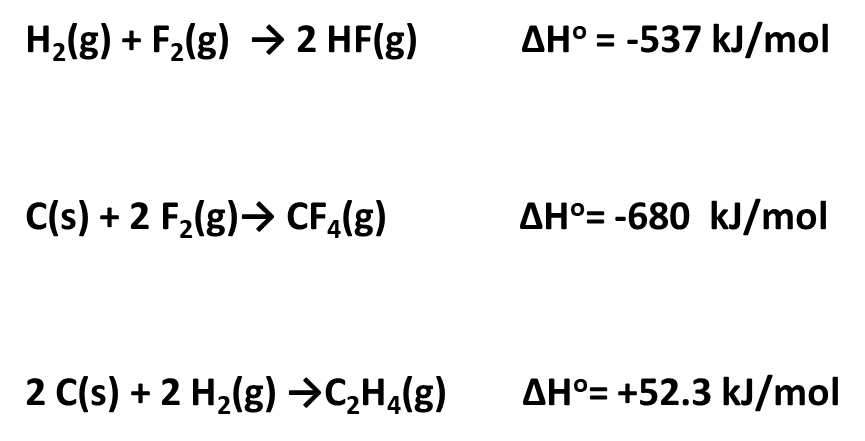
To determine the heat of reaction (ΔH) for this reaction, use Hess's Law with the provided ΔH° values for the reactions involved. You should add/subtract the appropriate ΔH° values from the reactions that lead to the formation of the products from the reactants.
Answer: Find ΔH∘\Delta H^\circΔH∘ for:
C2H4(g)+6F2(g)→2CF4(g)+4HF
Given Reactions:
H2(g)+F2(g)→2HF(g)ΔH∘=−537kJ
C(s)+2F2(g)→CF4(g)ΔH∘=−680 kJ
2C(s)+2H2(g)→C2H4(g)ΔH∘=+52.3 kJ
Modified Reactions:
Multiply (2) by 2: 2C(s)+4F2(g)→2CF4(g)ΔH=−1360 kJ
Multiply (1) by 2: 2H2(g)+2F2(g)→4HF(g)ΔH=−1074 kJ
Reverse (3): C2H4(g)→2C(s)+2H2(g)ΔH=−52.3 kJ
Final Calculation:
ΔH=−2486.3 kJ
Endothermic Reaction
A reaction that absorbs energy, characterized by a positive ΔH.
Hess's Law
The total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps.
Entropy (S)
A measure of the randomness or disorder of a system; increases with more microstates.
Second Law of Thermodynamics
The total entropy of an isolated system can never decrease over time; it only increases in spontaneous processes.
A thermodynamic potential that measures the maximum reversible work obtainable from a system at a constant temperature and pressure; G = H - TS.
ΔG < 0
Indicates a spontaneous process under given conditions.
ΔG > 0
Indicates a nonspontaneous process under given conditions.
State Function
A property whose value does not depend on the path taken to reach that specific value.
Standard State
The defined state of a substance at a temperature of 25°C and a pressure of 1 atmosphere.
S>0; s<0
increases entropy, vice versa
Molar Enthalpy of Formation (ΔHf°)
The change in enthalpy when one mole of a compound is formed from its elements in their standard states.
Free Energy of Formation (ΔGf°)
The change in Gibbs free energy when one mole of a compound is formed from its elements in their standard states.
Equilibrium Constant (K)
A number that expresses the ratio of the concentrations of products to reactants at equilibrium.
Reversible Reaction
A reaction that can proceed in both the forward and reverse directions.
Spontaneous Reaction Conditions
Conditions under which a reaction will favor products over reactants.
What do enthalpy vs time graphs represent in a chemical reaction?
Enthalpy vs time graphs show the changes in enthalpy (heat content) of reactants and products over the course of a chemical reaction, illustrating the energy absorbed or released.
What does the peak of an enthalpy vs time graph indicate?
The peak of an enthalpy vs time graph indicates the transition state, where the energy is at its maximum before the reaction proceeds to form products.
In an exothermic reaction, how do the energies of reactants compare to products on the enthalpy vs time graph?
In an exothermic reaction, the energy of the reactants is higher than that of the products, indicating that energy is released during the reaction.
In an endothermic reaction, how do the energies of reactants compare to products on the enthalpy vs time graph?
In an endothermic reaction, the energy of the reactants is lower than that of the products, indicating that energy is absorbed during the reaction.
How do you find activation energy (Ea) on an enthalpy vs time graph?
Activation energy (Ea) can be determined by measuring the height of the peak (transition state) from the level of the reactants' energy on the enthalpy vs time graph.
What does the activation energy (Ea) represent on an enthalpy vs time graph?
The activation energy (Ea) represents the minimum energy required for a reaction to proceed from reactants to products, shown as the difference in energy between the reactants and the transition state on the graph.
How do you find ΔH on an enthalpy vs time graph?
ΔH (change in enthalpy) can be found by calculating the difference in energy between the products and the reactants on the enthalpy vs time graph.
What does a positive ΔH value indicate on an enthalpy vs time graph?
A positive ΔH value indicates an endothermic reaction, where the energy of the products is higher than that of the reactants.
What does a negative ΔH value indicate on an enthalpy vs time graph?
A negative ΔH value indicates an exothermic reaction, where the energy of the products is lower than that of the reactants.
What is the formula for calculating ΔH of a reaction?
ΔH = Σ (ΔHf products) - Σ (ΔHf reactants), where ΔH_f is the standard enthalpy of formation for each substance involved in the reaction.
Provide an example of calculating ΔH for the combustion of methane (CH₄).
For the combustion of methane: CH₄ + 2 O₂ → CO₂ + 2 H₂O. The reaction ΔH = [ΔHf CO₂ + 2 × ΔHf H₂O] - [ΔHf CH₄ + 2 × ΔHf O₂]. Plugging in values from standard tables, ΔH can be calculated.
How do you predict relative entropy change (ΔS) for a reaction?
To predict relative entropy change (ΔS), consider the change in the number of moles of gas, phase changes, and temperature effects. Generally, if the number of gas molecules increases, ΔS is positive; if it decreases, ΔS is negative.
ΔS represents the change in entropy for a process. Entropy itself is a measure of the disorder or randomness in a system. A positive ΔS indicates an increase in disorder, while a negative ΔS suggests a decrease in disorder during a reaction or phase change.
Simply: S>0 Is an increase in entropy
S<0 is a decrease in entropy

Heating a pot of water → Positive entropy (Molecules move faster, increasing disorder).
Dissolving sugar in water → Positive entropy (Sugar molecules disperse, increasing disorder).
Making ice cubes → Negative entropy (Water molecules become more ordered in a solid).
Spilling a glass of milk → Positive entropy (Milk spreads out, increasing disorder).
Melting an icicle → Positive entropy (Solid ice becomes liquid, increasing disorder).
Provide an example of predicting ΔS for the reaction: 2 H₂(g) + O₂(g) → 2 H₂O(g).
In this reaction, there are 3 moles of gas (2 H₂ and 1 O₂) on the reactant side and 2 moles of gas (2 H₂O) on the product side. Since the number of moles of gas decreases from 3 to 2, ΔS is expected to be negative, indicating a decrease

A) He (g) → He (l) → Negative entropy (Gas to liquid decreases disorder).
B) Hg (l) (45°C) → Hg (l) (25°C) → Negative entropy (Lower temperature means lower molecular motion and disorder).
C) H₂O (l) → H₂O (s) → Negative entropy (Liquid to solid decreases disorder).
D) Ag⁺ (aq) + Br⁻ (aq) → AgBr (s) → Negative entropy (Ions in solution form a solid, reducing disorder).
E) I₂ (s) → I₂ (aq) → Positive entropy (Solid dissolving in water increases disorder).
Final Answer:Only E has a positive entropy change. ✅
How do you find the ΔS for a reaction?
To find the ΔS for a reaction, use the formula: ΔS = ΣS(products) - ΣS(reactants). Calculate the standard molar entropy values (S) for each product and reactant at standard conditions, sum them up for the products, sum them for the reactants, and then subtract the two sums to get ΔS.
given the following standard molar entropies, calculate delta S for the reaction
To find the entropy change (ΔS) for a reaction, use the formula:
ΔSrxn=∑Sproducts−∑Sreactants
Example:
Consider the reaction:
N2(g)+3H2(g)→2NH3(g)
Given standard molar entropy values (J/mol·K):
N₂ (g): 191.5
H₂ (g): 130.7
NH₃ (g): 192.5
Step 1: Calculate Total Entropy of Reactants Sreactants=(1×191.5)+(3×130.7)=191.5+392.1=583.6 J/mol
Step 2: Calculate Total Entropy of Products Sproducts=(2×192.5)=385.0 J/mol
Step 3: Calculate ΔS ΔS=Sproducts−Sreactants
ΔS=385.0−583.6=−198.6 J/mol
Conclusion:
Since ΔS is negative, this reaction leads to a decrease in entropy, meaning the products are more ordered than the reactants.
ΔHrxn∘=∑ΔHproducts∘−∑ΔHreactants
=[(4×90.3)+(6×−241.8)]−[(4×−45.9)+(5×0)]
= [(4 \times 90.3) + (6 \times -241.8)] - [(4 \times -45.9) + (5 \times 0)]
=[(4×90.3)+(6×−241.8)]−[(4×−45.9)+(5×0)]
=[(361.2)+(−1450.8)]−[(−183.6)+0]
=−1089.6+183.6=−906.0 kJ
Step 2: Calculate ΔS°surr ΔSsurr∘=−−906.0 kJ/298 K
=(906.0×10^3J)/(298 K)
=Final Answer: 3.04×103 J/K
give the formula for Delta S Surroundings
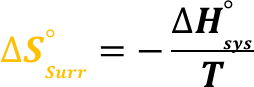
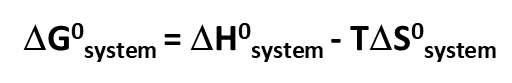
Gibbs free Energy and formula
the amount of free energy available to do work
DG < 0 for a spontaneous process
DG > 0 for a nonspontaneous process
DG = 0 for a process at equilibrium
Give the state function for Gibbs Free enrgy

what is delta G at 25*c if delta H is 177.8kJ and delta S = 160.7j/K
CaCO3(s) -> CaO + CO2(g)
To calculate ΔG° (Gibbs free energy change) at 25°C (298K), use the formula:
ΔG∘=ΔH∘−TΔS
Given Data:
ΔH° = 177.8 kJ = 177,800 J
ΔS° = 160.7 J/K
T = 25°C = 298 K
Step 1: Plug into the EquationΔG∘=177800−(298×160.7)
=177800−47888.6
=129911.4
=129.9 kJ
Consider a reaction that has a Positive delta H and a positive delta S. which of the following statements is TRUE?
The reaction is nonspontaneous only at high temps.
the reaction is spontaneous at high temps
this reaction will be spontaneous at all temps
this reaction will be nonspontaneous at all temps
it is not possible to determine without more information
The reaction is spontaneous at high temps;
Analysis:
At high temperatures (large TΔS), TΔS > ΔH, making ΔG negative → Spontaneous.
At low temperatures, TΔS is small, so ΔG may stay positive, making it nonspontaneous.
What are the signs of ΔH, ΔS, and ΔG for an ideal gas expanded at constant temperature?
For an ideal gas expanded at constant temperature:
ΔH (enthalpy change) = 0 (since it's isothermal and there's no change in internal energy for an ideal gas)
ΔS (entropy change) = + (entropy increases due to expansion)
ΔG (Gibbs free energy change) = 0 (at equilibrium and constant temperature, ΔG is zero).