Production of ethanol
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
What are the 2 types of production of ethanol
Hydration of ethane
OR
Fermentation
What is the reaction of hydration of ethene ?
Ethene + steam —> ethanol
What are the conditions for hydration of ethene ?
H3PO4 catalyst
300 *C
60 - 70 atm
What are the pro for hydration of ethene ?
Fast reaction
Continuous reaction
Pure final ethanol + 100 % atom economy
What are the cons of hydration of ethene ?
Ethene is non - renewable
High temp + pressure
$ process
Draw the mechanism of hydration of ethene
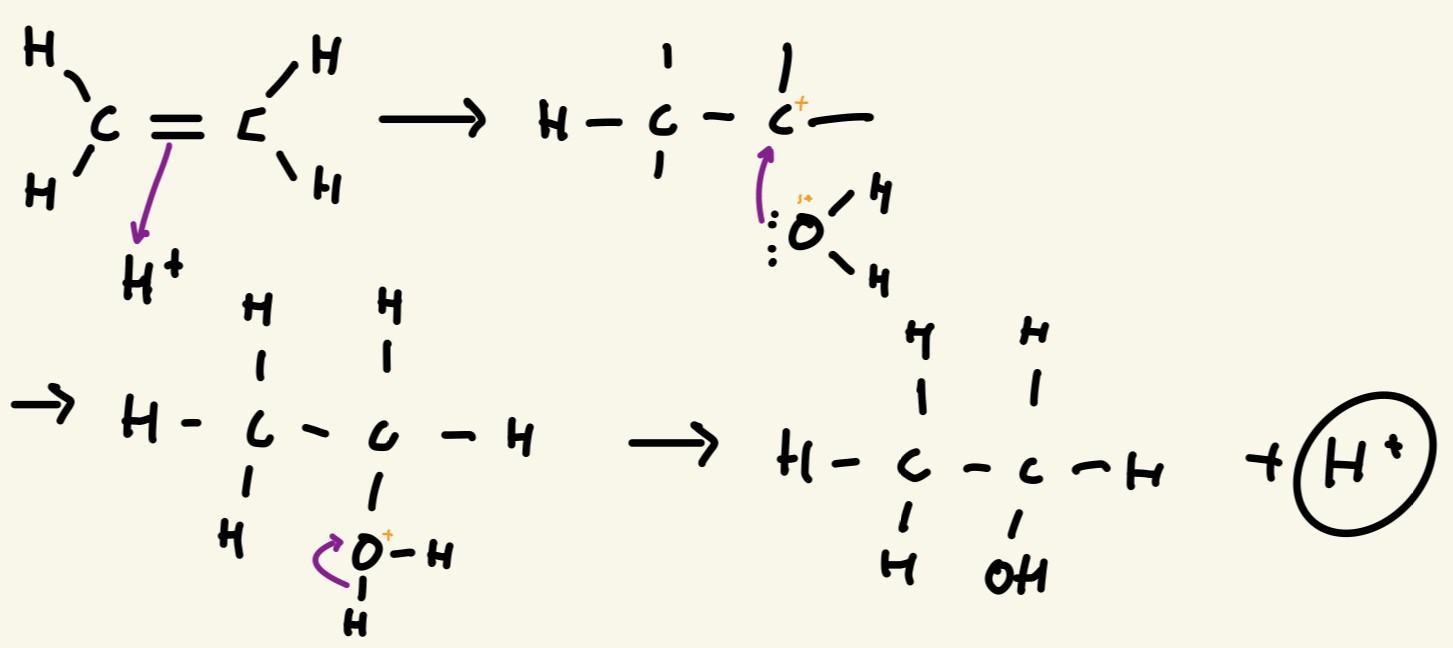
What happens in the process fermentation hint : glucose
Break down of glucose into ethanol + CO2 in anaerobic conditions
C6 H12 O6 —> Yeast —→ 2 C2 H5 OH + 2 CO2
What are the conditions of fermentation
Temp : 35 *c
Anaerobic conditions
Yeast enzyme
Why these conditions ?
35 *c → enzyme will denature if temp too high or temp too low reaction will be slow
Anaerobic : to prevent oxidation occurring ethanol + [ O ] → ethanoic acid
Yeast : replace the oxygen + allows for breakdown of glucose without oxygen
What are the pro of fermentation ?
Renewable
Low tech
Cheap
What are the conditions fermentation ?
Slow rate + 51.1 % atom economy
Batch process
need to distil to an ethanol
What does batch process means ?
Series of operation are accredited out over a period of time on a separate identifiable item or parcel of material
Where does Biofuels + fuels come from ?
Biological source
Biofuels + fermentation ?
It’s carbon neutral since the same amount of CO2 is absorb in photosynthesis of the plant and when we produce ethanol form the biofuels + burn the ethanol
Why is not all carbon neutral ?
But… fossil fuels will be have to burn to power machines to make fertilisers to harvest crops
And refinery equipment needs electric to power
And transport needs fuels to be burnt
What is the definition of of carbon neutral
there is a balance between carbon emissions and carbon absorption, resulting in a net zero carbon footprint.
How many of moles of CO2 is absorbed in photosynthesis
6 moles of CO2
6 CO2 + 6 H2O → C6 H12 O6 + 6H2O
How many moles of CO2 is released in fermentation of glucose
2 mole of CO2
C6 H12 O6 → 2 C2 H5 OH + 2 CO2
How many moles of CO2 is released in combustion of ethanol
4 mole of CO2
2 C2 H5 OH + 6 O2 → 4 CO2 + 6 H2O
How many moles of CO2 are totally released ? In fermentation + combustion
6 mole of CO2