Controlling air pollution
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Where are catalytic converters located?
Midway in the exhaust system of a vehicle between the engine and muffler
What are catalytic converters?
Converters with a coating of platinum, palladium and rhodium that speed up the conversion of harmful gases in the vehicle into less harmful susbtanaces
What are gases that leave an engine with a catalytic converter?
CO2, CO, N2, NO, NO2, O2, CxHy, H2O
Increased CO2 emissions, despite being air pollution, are preferred over the more harmful original pollutants
What are some redox reactions that can occur in a catalytic converter?
2CO (g) + 2NO (g) → 2CO2 (g) + N2 (g)
2CO (g) + O2 (g) → 2CO2 (g)
2NO2 (g) → N2 (g) + 2O2 (g)
2NO (g) → N2 (g) + O2 (g)
2NO2 (g) + 4CO (g) → N2 (g) + 4CO2 (g)
2C8H18 (g) [octane] + 25O2 → 16CO2 (g) + 18H2O (g)
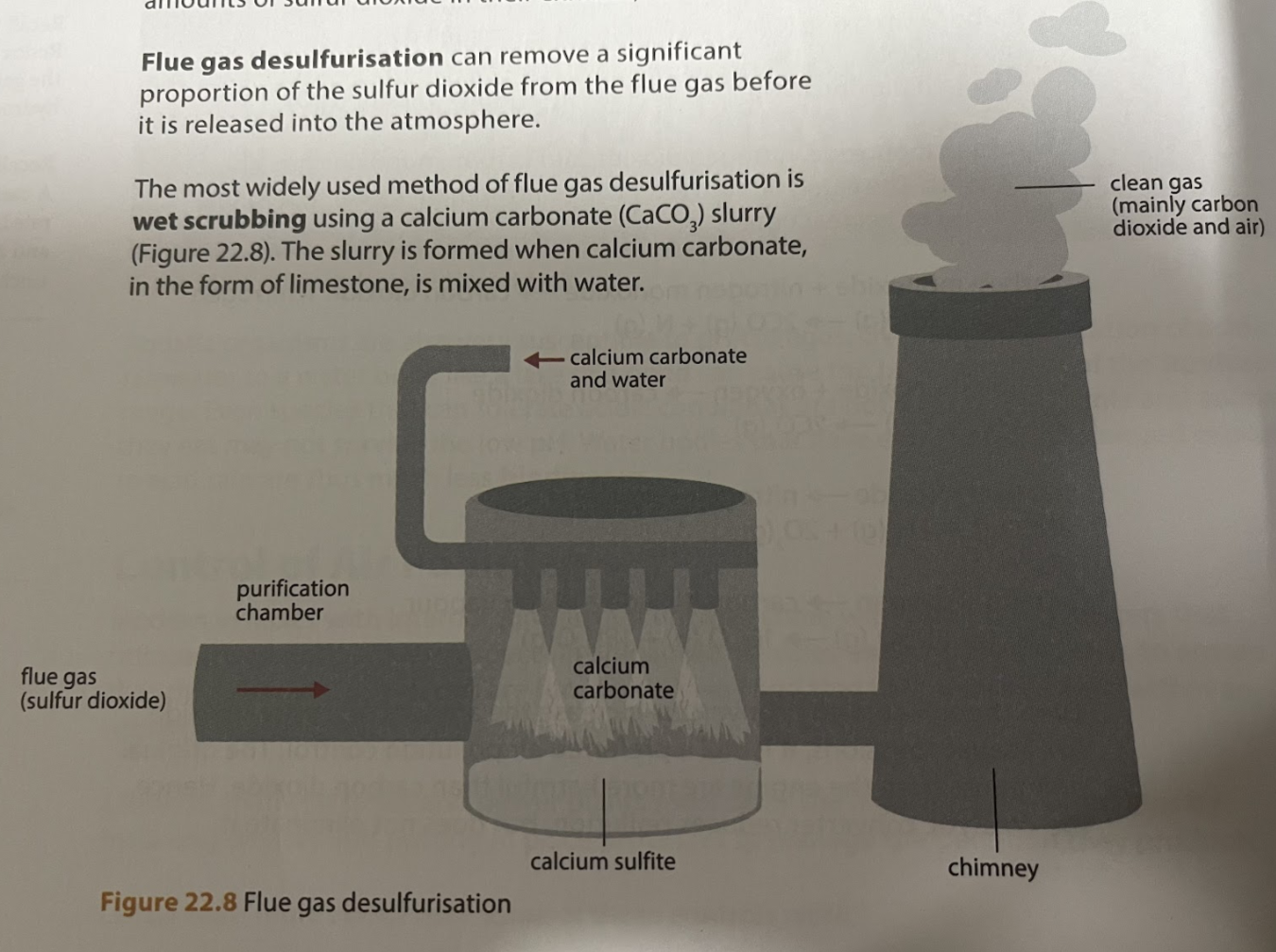
What does flue gas desulfurisation do?
Removes a significant portion of SO2 from flue gas before its release into the atmosphere
Why is it not practical to instal flue gas desulfurization set ups in vehicles?
They require a large amount of space
They can be quite costly to run
What is the most widely used method of flue gas desulfurization?
Wet scrubbing using CaCO3 (CaCO3 mixed with water) to form slurry
Why do fossil fuels have significant sulfur content?
From biological molecules in the organisms that gave rise to the fossil fuels
From sulfur compounds in the rocks surrounding the fossil fuel
How can excess sulfur be removed from fossil fuels?
Treat fossil fuels with hydrogen gas to produce H2S as a by-product, which allows for the production of low-sulfur petrol and diesel
What is the carbon cycle?
A set of processes that regulates the amount of carbon dioxide in the atmosphere
What happens during combustion of the carbon cycle?
Methane is burnt in the presence of oxygen to generate energy in an exothermic reaction
CH2 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
*Most fossil fuels contain mainly methane
What happens during photosynthesis of the carbon cycle?
Plants take in CO2 and H2O to make C6H12O6 and O2 in the present of sunlight in an endothermic reaction
Glucose is used in other processes that sustain plant life
6CO2 (g) + 6H2O (l) → C6H12O6 (s) + 6O2 (g)
What happens during respiration of the carbon cycle?
Living organisms gain the energy for life by breaking down glucose back into CO2 and water which are released into the atmosphere in an exothermic reaction
C6H12O6 (aq) + 6O2 (g) → 6CO2 (g) + 6H2O (l)
What happens during decomposition of the carbon cycle?
Dead organisms are broken down into simple compounds by decomposers
Aerobic decomposition in the presence of O2 releases CO2 into the atmosphere
Anaerobic decomposition occurs in the absence of oxygen, which is key to the formation of fossil fuels which trap carbon deep beneath the Earth’s surface
What happens during decomposition in the carbon cycle?
The ocean and other large bodies of water absorb CO2
Some marine plants use the dissolved gas to photosynthesise
Dissolved gas is also converted to carbonic acid H2CO3 and CaCO3, which makes up the shell of marine organisms and accumulate on the seabed when the organisms die
What is liming?
CaCO3 is added to soil and water bodies to remove some of the excess acid contributed by acid rain
What are the limitations of liming?
Acid rain affects vast areas
There are limits to regions that can be protected, such as territorial boundaries
Expensive process with temporary effects
What is ozone (O3)?
A gas that occurs in both the Earth’s upper atmosphere and at ground level. At ground level, ozone is a harmful pollutant
How do CFC (chlorofluorocarbons) destroy the ozone layer?
UV radiation from the sun breaks up CFC molecules in the stratosphere into chlorine atoms
Within the ozone layer, chlorine atoms react with ozone to form O2 and ClO
ClO continues to break up another ozone molecule
Cl atom is released and continues to attack another ozone molecule in a chain reaction
Holes in the ozone layer begin to form, allowing harmful UV radiation to reach the Earth’s surface
What happens during flue gas desulfurisation before CaSO4 is hydrated?
Slurry droplets are sprayed through flue gas and Ca
Describe the process of flue gas desulfurization
CaCO3 (s) + SO2 (g) -> CaSO3 (s) + CO2 (g)
Slurry droplets are sprayed through the flue gas and the CaCO3 reacts with the sulfur dioxide to form calcium sulfite and CO2 gas
NO2 can also react with CaCO3 and will be removed
2CaSO3 (s) + O2 (g) -> 2CaSO4 (s)
Calcium sulfite is then further oxidised to form CaSO4 by atmospheric O2
CaSO4 (s) + 2H2O (l) -> 2CaSO4 2H2O (s)
CaSO4 can then be hydrated to form hydrated calcium sulfate, also known as gypsum
Gypsum produced can be used to make drywalls, fertilisers, plaster for sculptures and casts that stabilise fractured bones