Drug affinity (Receptor theory 1), L2
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Explain the term ligand
molecule that binds to a specific receptor site on another molecule. This binding may or may not trigger a biological response.
Explain the term affinity
tendency for a drug to bind to a receptor, drugs of high potency generally have a high affinity for their receptors
Explain what Kd is
measure of the affinity of a ligand for its receptor, represents the concentration of ligand at which half of the receptors are occupied
What is the most basic test for whether a binding reaction has reached equilibrium?
fraction of complex formed between two molecules does not change over time - as many complexes are formed as dissociated
How do you find Kd ? (equation)
rate of reverse reaction over rate of the forward one
KD= k-1 / k+1 = [Aeq]*[Req]/ [AeqReq]
What is the unit of Kd ?
moles
What is the efficacy of agonists vs antagonists? Define efficacy
significant efficacy ie over 0 // 0. tendency for a drug, once bound, to activate a receptor
Explain the concept of receptor occupancy
proportion of receptors occupied will vary with the drug concentration, varies between 0 and 1 (all receptors occupied)
Name some quantitative methods used in pharmacology to measure drug affinity
radioligand binding assays
fluorescence polarisation assays
surface plasmon resonance
isothermal titration calorimetry
computational modelling
Name some limitations and potential problems encountered when performing drug-binding experiment
Radioligand binding assays: Requires radioactive materials, may not be suitable for all receptors.
FPA: Requires a fluorescently labelled drug, may be less sensitive than radioligand binding assays.
SPR: Real-time measurement, can be used for both small and large molecules, label-free.
ITC: Requires specialized equipment, may be less sensitive for low-affinity interactions.
Modeling: Requires computational expertise, may not always accurately predict experimental results.
What is a similarity between agonists and antagonists?
both bind to receptors
What is a difference between agonists and antagonists?
only agonists activate the receptors to induce signalling and a response
What is the forward rate of a reaction?
k+1[A]*[R]
What is the reverse rate of a reaction?
k-1 [AR] , governed by the disassociation rate
What governs occupancy? What values does the latter vary between?
affinity
Occupancy varies between 0 (no drug present) and 1 (all receptors occupied)
Define occupancy
proportion of receptors occupied will vary with the drug concentration
Equation to determine occupancy
nb of receptors occupied / total nb of receptors ie [AR]/[R]
When does measuring/quantifying biological responses to a drug not necessarily work?
when studying an antagonist bc by def doesn’t produce measurable biological reaction
What is the relation between affinity and occupancy?
High affinity generally leads to higher occupancy
Briefly explain the principle of Radioligand Binding Assays to measure drug affinity
A radiolabelled drug is incubated with receptor-containing tissue or cells. The amount of radiolabel bound to the receptors is measured.
Briefly explain the principle of Fluorescence Polarization Assays (FPAs) to measure drug affinity
A fluorescently labelled drug is used. When it binds to a receptor, its rotational freedom decreases, leading to increased polarization of emitted fluorescence.
Briefly explain the principle of Surface Plasmon Resonance (SPR) to measure drug affinity
Measures changes in the refractive index of a surface when molecules bind to it.
Briefly explain the principle of isothermal titration calorimetry (ITC) to measure drug affinity
Measures the heat released or absorbed during a binding interaction.
Briefly explain how computational modelling can be used to measure drug affinity
Uses computational methods to predict drug-receptor interactions based on molecular structures.
Name four practical considerations for measuring receptor affinity
source of receptors and incubation conditions (tissue/cells with correct receptors, incubation must conserve ligands and receptors)
radioligand (pure, degradation, labelling)
separating bound from free (filtration or centrifugation, dialysis, column chromatography - rate is main consideration)
non-specific binding (reduced by anti-absorbants like albumin, collagen for peptides, o-catechol for catecholamines)
How is non specific binding determined?
by addition of excess non-radioactive drug bc Specific ‘bound’ radioactive drug which is present in lower concentrations will be displaced, non-specific bound radioactive drug will remain
What are advantages and disadvantages of 3H as a radio label?
+: Labelled product indistinguishable from native compound; High specific activities (>80 Ci/mmol) can be obtained; Good stability when properly stored; Long half-life (~ 12.5 years)
-: Specialized labs required; Labelling is expensive & difficult
What are advantages and disadvantages of 125I as a radio label?
+: If compound has an aromatic hydroxyl group (eg. tyrosine residues in peptides) can be incorporated at very high specific activities (> 2000 Ci/mmol); Iodination easy in most labs and cheap
-: More readily degraded; Biological activity of ligand can be reduced; (ie. not functionally "invisible"), Short half life (67 days)
What are types of ligands used in radioligand binding assays?
neurotransmitters, hormones, growth factors, cytokines/chemokines, drugs, toxins
What are types of protein targets in radioligand binding assays?
receptors, ion channels, enzymes, carrier molecules
How do you find the amount of specific binding that occurs for a ligand and its receptor?
Specific binding = Total bound – nonspecific binding
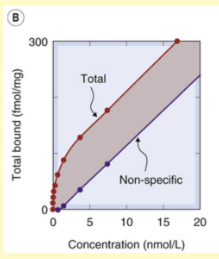
What does the following graph of radioligand binding show about the limits of non specific binding?
there is no limit, non specific binding is infinite
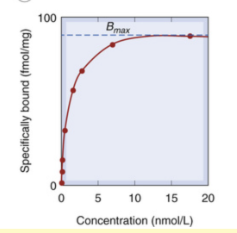
What does the following graph of radioligand binding show about the limits of specific binding?
once all receptors are occupied, they are termed “saturated“ and the limit of specific binding is reached
What type of scale is binding data usually plotted on?
a semi-logarithmic scale
Specific Binding shows ‘saturation’ while non-specific does not. Why?
specific binding relies on specific receptors - once all of these are occupied, they are saturated. non specific binding refers to all other types of binding ie can even be to the tube, etc so there can always be more
Why is it recommended when doing experiments on binding data in multiples of 1 and 3?
binding data is usually plotted on a semi-logarithmic scale so multiples of 1 and 3 are easier to read on that type of plot
Which equation shows the relationship between receptor occupancy, affinity and drug concentration?
langmuir/scatchard equation
If a drug has high affinity for its receptor, will the concentration of the drug needed be higher or lower? And its Kd?
lower
lower
When a drug has high affinity for its receptor, will the forward or the reverse reaction be faster?
forward ie more complexes made than dissociated
Describe the binding capacity for a drug
B max is capacity - saturation of receptors ie every receptor is bound to a ligand, forming a complex AR = no more can be made
What is Kd a measure of? What is it equivalent to?
Kd is measure of affinity
Kd is equivalent to concentration of drug required to occupy 50% of receptors at equilibrium
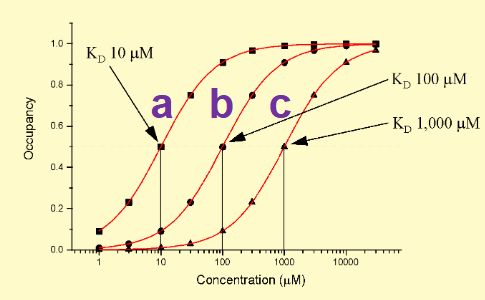
On the following graph, which drug has the highest affinity for the receptor? What shows this?
drug a bc lowest Kd = lowest amount needed to reach 50% occupancy ie highest affinity
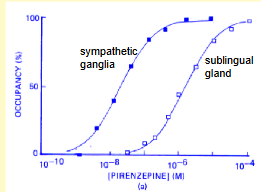
What does this graph show about the affinity of the drug for muscarinic receptors in sympathteic ganglia and sublingual gland resepctively? What does this tell us abut the structure of muscarinic receptors?
drug is clearly specific for muscarinic receptors in the symathetic ganglia (less drug needed to reach Kd) ie muscarinic receptors have different structure subtypes - drug can bind to both but binds better to the SG receptor
How was it first discovered that muscarinic receptors have different subtypes?
experimentally by pharmacologists testing drugs for affinity with various receptors like muscarinic ones - different affinity to a same ACh ligand implies different subtypes exist. later confirmed by new molecular techniques
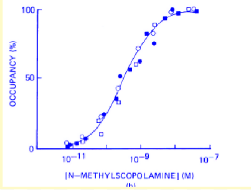
How does this curve show an absence of selectivity of the ligand for the receptors studied?
both ligands/drugs bind to the receptors at the same rate - one doesn’t have a higher affinity than another
Define ligand competition
inhibitor competes with the ligand for the same binding site on the receptor
What is the inhibition constant?
Ki is a measure of the potency of a competitive inhibitor in preventing the binding of a ligand to its receptor.
What does a lower Ki value indicate?
more potent inhibitor - better competition against other ligands
In competitive inhibition, where the inhibitor competes with the ligand for the same binding site on the receptor, what is the relationship between Ki and Kd?
Ki ≈ Kd
inhibition constant (Ki) is approximately equal to the dissociation constant (Kd) of the ligand because the inhibitor competes with the ligand on a one-to-one basis.
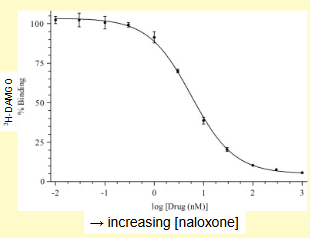
Explain what the following graph demonstrates about the the result of increasing amounts of naloxone being added to mu-opioid receptors already bound to H-DAMGO
with increasing concentrations of naloxone, the % of ‘bound’ 3H-DAMGO decreases ie. It is displaced from the receptor binding sites - example of ligand competition
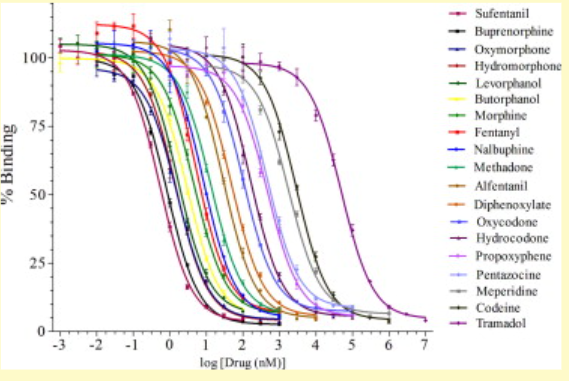
Which opiate has the highest affinity for the mu opioid receptor?
Which has the lowest affinity?
sufentanil (curve most to left)
tramadol (curve most to the right)
If a drug is made with a high affinity, how would this effect the concentration of antagonist needed to displace them?
high concentrations of antagonists would also be needed to displace the agonist
What important aspects of drug function does the affinity and potency of drugs not inform on?
this tells you nothing about the action of the drug in terms of cell signalling and physiology!
What Law does the binding of drugs to receptors obey? Explain
Law of Mass Action
rate of a reaction is proportional to the product of the concentrations of each reactant (duh)
What 2 factors does agonist potency depend on?
affinity and efficacy
What does the binding of an agonist to its receptor trigger?
biological response ie change in cell function
Explain potency
amount of drug needed to produce a given effect
What distinguishes potency from efficacy?
amount of drug needed for an effect // max possible effect
Does a partial or full agonist have higher efficacy?
full agonist, partial agonist has lower efficacy (it can't produce the same max response as a full agonist)
Does a partial or full agonist have higher potency?
depends, a partial agonist can bind to the receptor with higher affinity, allowing it to achieve a larger effect at low concentrations than a less potent full agonist - just won’t be able to achieve max response