Module 3: Bacteriology
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
69 Terms
What is microbial virulence
It is the degree to which a microorganism can cause damage to a host organism
How can microbial virulence be measured
By determining the LD₅₀ (dose that kills 50% of test animals). Highly virulent pathogens require only a small difference in dose to kill 50% vs 100%.
What are the main roles of virulence factors?
To aid colonisation and help bacteria evade immunity and damage the host tissue
Give an example of a model microorganism used to study virulence.
Staphylococcus aureus, E. coli, Shigella, Helicobacter pylori.
How does normal flora compete with invading pathogens
by occupying niches , producing inhibatory compounds, competeing for nutrients and stimulating host defenses
What are the 6 ways virulence factors promote colonisation
1) Adhere to host cells (pili, adhesins), 2) Invade host cells (invasins), 3) Contact host cells (motility/flagella), 4) Resist phagocytosis (capsule), 5) Evade immune defences (phase/antigenic variation), 6) Compete for nutrients (siderophores).
Example of pili aiding colonisation?
Uropathogenic E. coli pili adhere to urinary tract epithelium → UTI.
Example of invasins?
Shigella invasins allow entry into colonic epithelial cells.
Example of motility aiding colonisation?
Helicobacter pylori uses flagella to move through gastric mucus
How does Streptococcus pneumoniae resist phagocytosis?
By producing a polysaccharide capsule - the capsule obscures the bacterial surfaces make it harder to reccognise and phagocytose.
How do bacteria evade immune defences?
Capsule mimicry (e.g. sialic acid in N. meningitidis, hyaluronic acid in S. pyogenes), or antigenic variation (e.g. N. gonorrhoeae).
How do bacteria compete for nutrients?
They secrete siderophores, which bind iron tightly.
WHat are the to broad classes of toxins
Endotoxins ( LPS lipid A from Gram−ve bacteria) and Exotoxins (secreted proteins)
What symptoms do endotoxins cause?
Fever, shock, sepsis (same symptoms regardless of species).
What are exotoxins?
Soluble toxins secreted by bacteria (Gram+ or Gram−) that disrupt host functions.
What are the 3 main types of exotoxins?
Cytotoxins (kill/inhibit cells), Neurotoxins (disrupt nerve transmission), Enterotoxins (act on gut epithelium).
How can diseases caused by exotoxins be prevented?
By neutralising with antibodies (antitoxin) or vaccination with toxoids (inactivated toxins).
What structural defenses does S Aureus have
Protein A (binds antibodies to evade detection), capsules, adhesins
What is MRSA?
Methicillin-resistant Staphylococcus aureus, resistant to many β-lactam antibiotics.
What is VRSA?
Vancomycin-resistant S. aureus — very difficult to treat.
Why is S. aureus a successful pathogen?
It has multiple virulence factors (adhesins, capsule, enzymes, exotoxins) that aid colonisation, immune evasion, and tissue damage.
What two key concepts are illustrated using Group A Strep?
Koch’s molecular postulates (updated from previous)
Redundancy in virulence factor profiles
What is another name for Group A Streptococcus?
Streptococcus pyogenes
Where does it commonly colonize?
Throat and skin
What type of bacteria is it?
Gram-positive cocci with beta-hemolysis on blood agar
What is the most common disease caused by S. pyogenes?
Pharyngitis ("strep sore throat")
What are two localized infections caused by S. pyogenes?
Cellulitis and impetigo
Name three invasive infections caused by S. pyogenes.
Bacteraemia, toxic shock syndrome, necrotising fasciitis
WHat is post-streptococcal sequelae
theyre symptoms they remain after being infected with strep A multiple times.
What are examples of post-streptococcal sequelae
Acute glomerulonephritis (kidney failure)
Acute rheumatic fever (heart failure)
What triggers rheumatic heart disease
An inappriate Immune response against M protein of S. pyogenes - immune system whinks it is attacking the microbe when it is actually attacking the heart cells
Why does M protein cause cross-reactivity?
It shares structural similarity with heart myosin (coiled-coil alpha helices)
What technique is the gold standard for pathogen categorization?
Pulse Field Gel Electrophoresis (PFGE)
What does PFGE help determine?
Whether outbreaks come from one or multiple sources - is it flesh eating or sore throat streptococcal
Why might prophylactic antibiotics fail in endemic regions?
Unavailability or needle avoidance
What is the goal of vaccine research for S. pyogenes?
Develop one-shot vaccines that prevent infection without causing sequelae
What are Koch’s original four postulates?
Bacteria present in every case
Isolated and grown in pure culture
Disease reproduced in healthy host
Same bacteria recovered from host
Why might Koch’s postulates fail?
Some bacteria can't be cultured (e.g., M. leprae)
No animal model available
What do molecular postulates focus on?
Identifying genes responsible for virulence
What are the key criteria for molecular postulates?
Gene present in virulent strains
Absent in avirulent strains
Disruption reduces virulence
Complementation restores virulence
Expression in vivo - (in the body)
Immune response protects
What is the second virulence factor foer GAS (Group A Strep.)
Fibronectin Binding Proteins (FBP) - Allow GAS to bind to the Fibronectin on the cell surface of our cells. Different GAS strains can be attributed to different combination so fthe many FBPs which there are - explain the broad spectrium of strep A diseases
What was the old “excess acid hypothesis” for ulcers?
Ulcers were thought to be caused by excess stomach acid, linked to stress, age, and diet.
Who discovered H. pylori’s role in ulcers?
Barry Marshall and Robin Warren (Nobel Prize, 2005).
What is the Gram status and shape of H. pylori?
Gram-negative, spiral-shaped bacterium with multiple flagella.
How is H. pylori mainly transmitted?
Person-to-person, mainly oral-oral route (via belching or reflux).
What serious cancer is strongly linked to H. pylori infection?
Gastric cancer (second leading cause of cancer death worldwide).
Where does H. Pylori inhabit
the gastric mucosa - they are in close proximity to the gastric epithelial cells
What percentage of duodenal ulcers are linked to H. pylori?
About 90%.
What are the virulence factors associated with H. Pylori
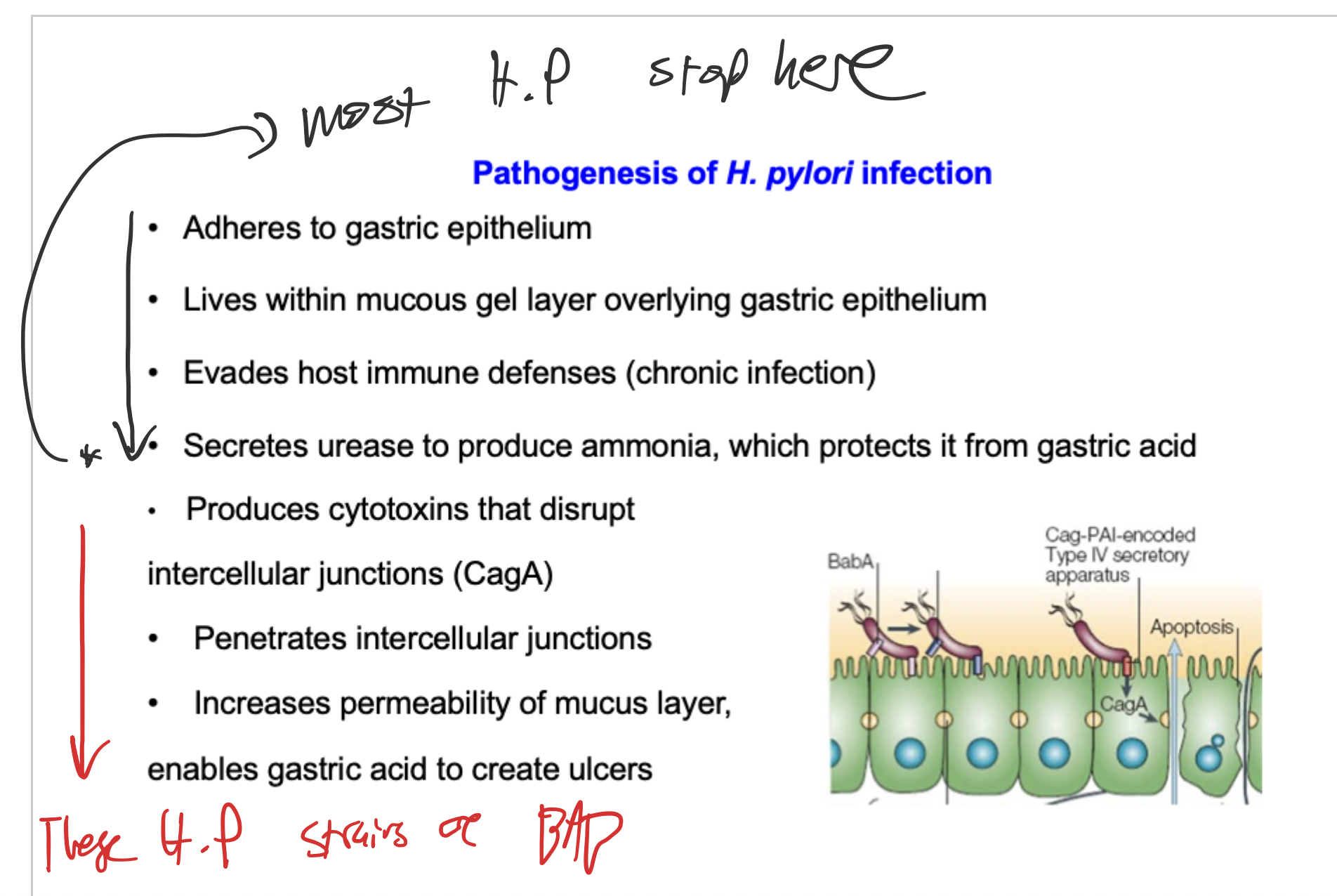
Urease - neutralises gastric environment by creating ammonia , flagella - allow penetration of mucous layer, adhesins (BabA, SabA) - binding ot host cells , mucinase - degrades gastric mucus , CagA.
Urease
Increases pH by releasing ammonia. Ure1 is a transporter protein requred by Urease
What is the main test for H Pylori
rapid urease test - breath test
WHat is the difference in H Pylori stains which cause no symptoms, and ones which do
CagA pathogenicity island is an example. It is injected into the eptithelial cells by type 4 secretion system - causes host cell inflammation.
Pathogenesis of H. Pylori
What is the main treatment for H. pylori infection?
Combination therapy: acid-lowering drugs + antibiotics (amoxicillin, tetracycline, metronidazole).
Is H. pylori always pathogenic?
No – some infections are asymptomatic, and it may protect against oesophageal adenocarcinoma.
How are new genes so easily introduced into bacterial genomes without disrupting processes
bacteria are able to accept new genes bacause of how easily they are able to divide and grow.
how do bacteria spread their antibiotic resistance
Conjugation, transposition, integrons, gene cassettes
Plasmids
circular pieces of DNA which are the reason bacteria can spread resistance so quickly - involved in conjugation
transposable elements
dna sequences which can jump from one position to another or from one dna molecule to another - the two major types are transposons and insertion sequences
Insertion sequences
smallest and simplest transposable elements. Has a gene encoding the transposase protein. DNA which contains the info to cut and copy itself in a new location
transposons
made up of an insertion sequence+extra genes. these extra genes are often made of antibiotic resistance genes. they can insert into chromosomal DNA or plasmids. if one is inserted into a conjugative plasmid (one which can be transferred between bacteria) then the transposon can also be spread.
what are the two mechanism of transposition
Conservative transposition: Cut/Paste - transposable element is excised from one location and reinserted. Replicative Transposition: copy/paste - new copy of transposon is produced and inserted in new location
conservative transposition (e.g. IS1)
Transposase makes staggered nicks in target DNA.
The IS element integrates into the nicked site.
Host DNA polymerase and ligase fill the gaps.
This generates direct repeats of the target sequence flanking the inserted IS element.
replicative transposition (e.g., Tn3).
Replicative transposition makes a copy of the transposon instead of moving it.
In Tn3:
Transposase links donor and target DNA → forms a cointegrate.
DNA replication duplicates the transposon.
Resolvase separates donor and target DNAs.
👉 Both DNAs end up with a copy of Tn3.
How do transposons acquire multiple antibiotic resiatance genes
By replicative transposition and recombination — transposons can pick up resistance genes when they move between plasmids and chromosomes, and can integrate additional resistance cassettes or other transposons, creating composite transposons with multiple genes.
Integron
It is found within a transposons and is a genetic unit capavbe of the capture and expression of genes that are contained in mobile elements called gene cassetes. It also provides a promoter - thus acting as natural cloning and expression vector for gene cassettes
Essential features of integrons
Attachment site - recognised by integrase and acts as acceptor site for cassettes.
A site specific recombinase enzyme (cuts and rejoins specific dna sequences) which is responsible for inserting/removing gene cassettes as the chosen region
Promoter - drives expression of inserted sequence
Approaches to reduce ths spread of antibiotic resistance
stop innapropriate use of antibitotics to reduce selective pressure for antibiotic resistance. remove ineffective antibiotics from use.