Nutrition Ch 9 - LOs
1/69
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
70 Terms
metabolism
the chemical processes and reactions involved in maintaining life
enable us to release energy from carbs, fat, protein, and alcohol
permit us to synthesize new substances and excrete waste products
metabolic pathway
group of reactions that occur in a progression
anabolic pathway
use small compounds to build larger ones
building blocks: glucose, fatty acids, cholesterol, and amino acids
requires energy
more prominent during growth
catabolic pathway
break down compounds
ex. glycogen » glucose
release of CO2, H2O, and ATP
more prominent during weight loss or wasting disease
adenosine triphosphate (ATP)
energy source used to:
synthesize new compounds
contract muscles
conduct nerve impulses
pump ions across membranes
how does ATP generate energy?
high energy bonds between phosphates are broken during hydrolysis
add phosphate(s) back to AMP and ADP
how ATP is regenerated and recycled by cells
oxidized
loses electrons/H
gains O
reduced
gains electrons/H
loses O
derivatives of niacin and riboflavin
transfer hydrogens and energy yielding compounds to oxygen in metabolic pathways
niacin (vitamin B-3)
component of nicotinamide adenine dinucleotide
NAD+
what is the oxidized form of niacin?
NADH
what is the reduced form of niacin?
riboflavin (vitamin B-2)
component of flavin adenine dinucleotide
FAD
what is the oxidized form of riboflavin?
FADH2
what is the reduced form of riboflavin?
pantothenic acid (vitamin B-5)
precursor to coenzyme A (CoA)
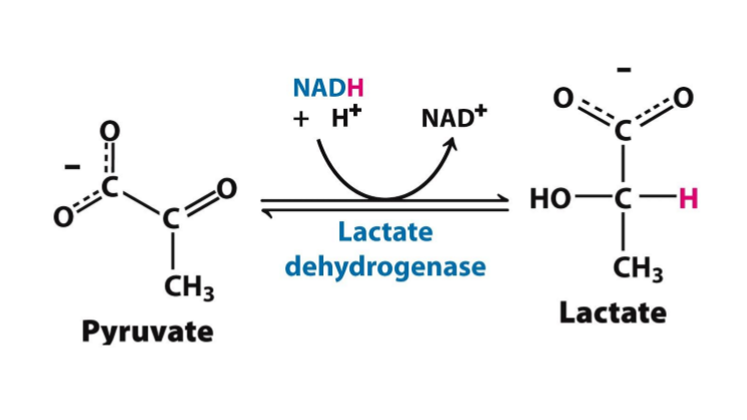
pyruvate»lactate
what is being reduced (gain H) in this reaction?
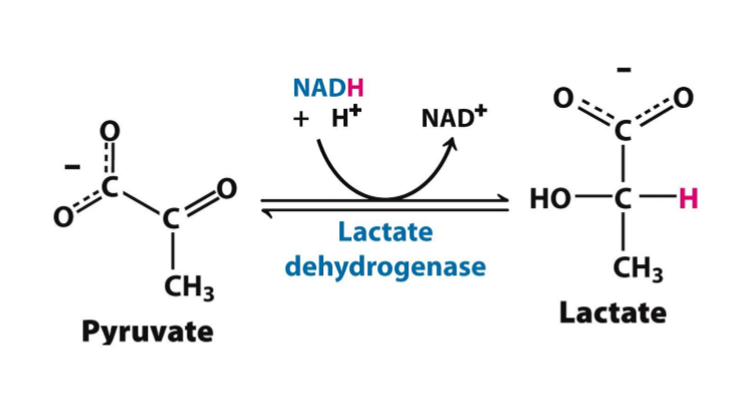
NADH»NAD+
what is being oxidized (lose H) in this reaction?
aerobic cellular respiration
molecules from food are oxidized to form ATP with O2 as final electron acceptor
30-32 ATP yield
anaerobic metabolism
insufficient O2 present
incomplete breakdown of glucose
creates 2 ATP per glucose
glycolysis
INPUT: 1 glucose
OUTPUT: 2 pyruvate, 2 NADH & H+, 2 ATP
occurs in cytosol
role is to break down carbs to create energy and create building blocks for anabolic pathways
doesn’t need oxygen
transition reaction
INPUT: 2 pyruvate
OUTPUT: 2 Acetyl-CoA, NADH & H+, CO2
occurs in mitochondria
requires oxygen
irreversible rxn
citric acid cycle
INPUT: 2 Acetyl-CoA
OUTPUT: 6 NADH & H+, 2 FADH2, 2 GTP (to make ATP), CO2
electron transport chain
INPUT: NADH & H+, FADH2
OUTPUT: ATP, water, NAD+
oxidative phosphorylation (O2 final acceptor) » combine with protons to form water, regenerates NAD+
- carried by NADH+ & H+, and FADH2 to form ATP
- needs Cu and Fe
fermentation
pyruvate is produced during glycolysis and converted to lactate » meant to regenerate NAD+ to keep glycolysis going (slow generation of ATP)
less glycolysis
what occurs when there is less NAD+?
cori cycle
generates lactic acid back into glucose in muscle fibers
steps of fatty acid metabolism
lipolysis
fatty acid oxidation
carnitine shuttle
beta oxidation/fatty acid breakdown
TCA cycle
ETC
lipolysis
breakdown of triglycerides into free fatty acids and glycerol
fatty acid oxidation
breakdown of fatty acids for energy production
fatty acids broken down with O2 as electron acceptor
occurs in the mitochondria
carnitine shuttle
fatty acids are taken up by cells and shuttled into mitochondria from cytosol
hormone sensitive lipase
during fasting, triglycerides from adipose tissue are broken down into fatty acids
activity is increased by glucagon, growth hormone and epinephrine
activity is decreased by insulin
oxaloacetate
combines with acetyl-CoA to create citrate in TCA
beta oxidation
fatty acid carbons are cleaved off in pairs » produce NADH and FADH2
cleaved carbons make acetyl-CoA, which enters TCA
TCA
fatty acids have more carbons than glucose:
Glucose: 2 turns of TCA
Fatty acids: 1 turn per carbon pair (2-26 pairs per acid)
Fatty acids store more chemical energy than glucose (less oxygen)
Fats yield more energy than carbs (9 vs. 4 kcal)
some TCA compounds are used for other purposes
cells that use glucose-derived pyruvate replenish oxaloacetate (keeps TCA for fatty acids running)
there is no pathway to make carbs from fatty acids
how do carbs aid fat metabolism?
“fats burn in a carbohydrate flame”
fats are more efficiently utilized than carbohydrates
production of ketoacids
results in metabolic acidosis (depletion of bicarbonate)
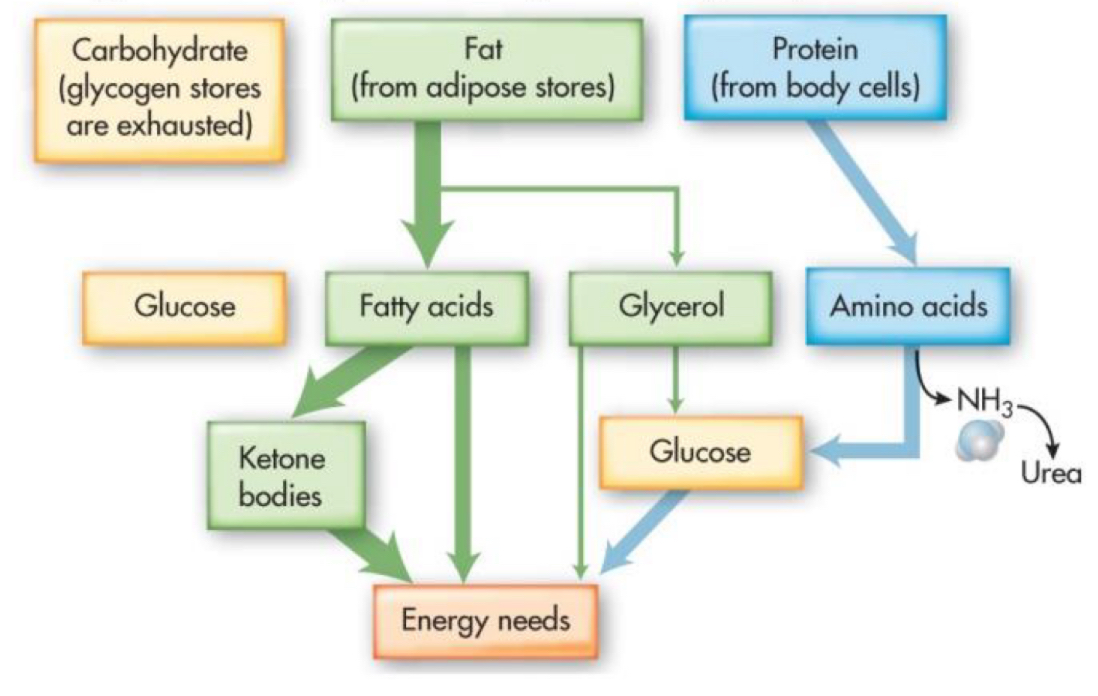
fasting ketosis
acidosis is mild
self limiting: ketosis increases insulin sensitivity results in decreased fatty acids release and increased glucose uptake
diabetic ketoacidosis
more serious metabolic acidosis
seen in type 1 diabetics with insulin deficiency
glucogenic amino acids
use carbons from carbon skeleton to form glucose
ketogenic amino acids
use carbons from acetyl-CoA
amino acids being used for fuel must first be deaminated
require vitamin B
results in carbon skeleton entering TCA, creates acetyl-CoA OR pyruvate
gluconeogenesis
allows body to create glucose from breakdown products of carbs, fats, or proteins
WHERE: mainly in the liver, partly in the kidneys
STARTING: glycerol, glucogenic AA, lactate » pyruvate (cori cycle), oxaloacetate
VITAMINS: B
glycolysis and the transition reaction create acetyl-CoA which enters the TCA
how do carbs convert into energy?
lipolysis breaks down fatty acids » pyruvate » acetyl-CoA » TCA
how do fats convert into energy?
deamination breaks down proteins to AA » carbon skeletons of AA are converted into Acetyl-CoA or into other intermediates (oxaloacetate, fumarate) that enter the TCA
how do proteins convert into energy?
alcohol dehydrogenase converts ethanol to acetaldehyde » acetate » add CoA » Acetyl-CoA, which enters TCA
how does alcohol convert into energy?
carbon skeletons, amino acids, proteins, ammonia & urea
what can amino acids convert to?
stored body fat and glucose
what can glycerol convert to?
glucose
what can glycogen and glucose convert to?
stored body fat, cholesterol, and VLDL
what can fatty acids convert to?
stored body fat
what can alcohol convert to?
liver
what organ plays a major role in metabolic pathways
ATP concentrations
enzymes
hormones
what does regulation involve?
gluconeogenesis
protein breakdown
lipolysis
low levels of insulin promote
glycogen
fat
protein
increased insulin promotes synthesis of
absorptive state
what is another term for feasted state?
post-absorptive state
what is another term for fasted state?
fasting
energy sources vary depending on length
depletion of lean mass to 50% is fatal
approx. 7-10 weeks
depletion of electrolytes due to diuretic effects of ketones
buildup of urea due to protein catabolism
feasting
excess energy intake from any source that results in fat storage
excess dietary fat can be stored with minimal processing
excess carbs
maximize glycogen as energy
convert to fat, spare fat from lipolysis
excess protein
mostly convert to glucose
small amount of storage
increased muscle protein synthesis if combined with intense exercise
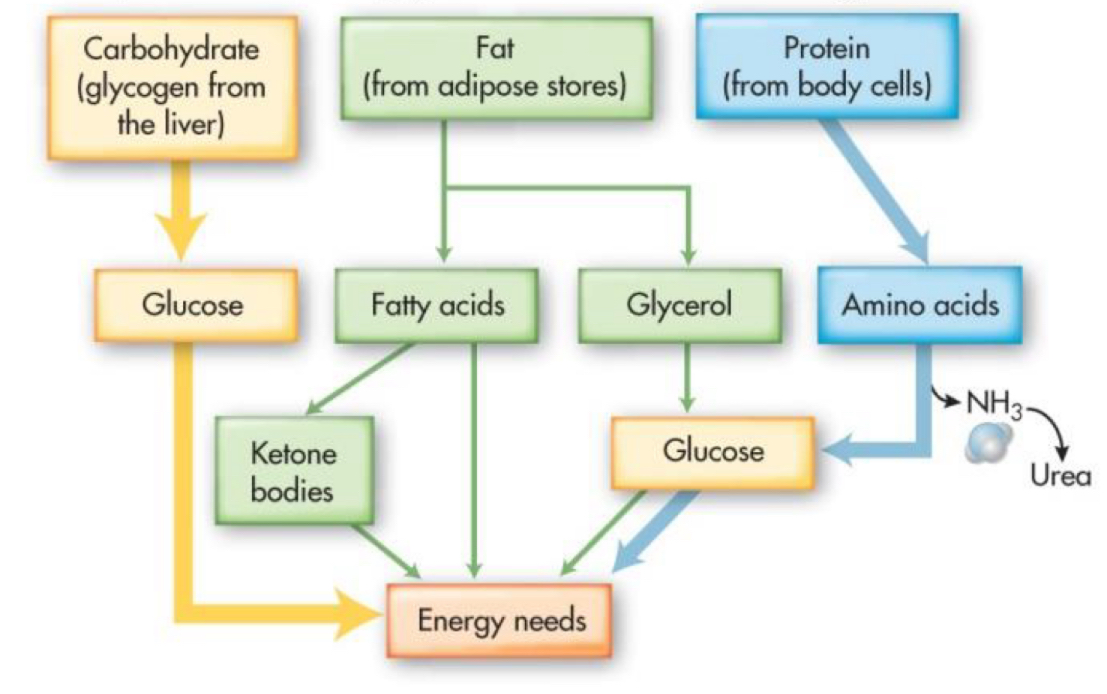
0-6 hrs after eating (postprandial fasting)
energy utilization:
carbs
proteins
fats
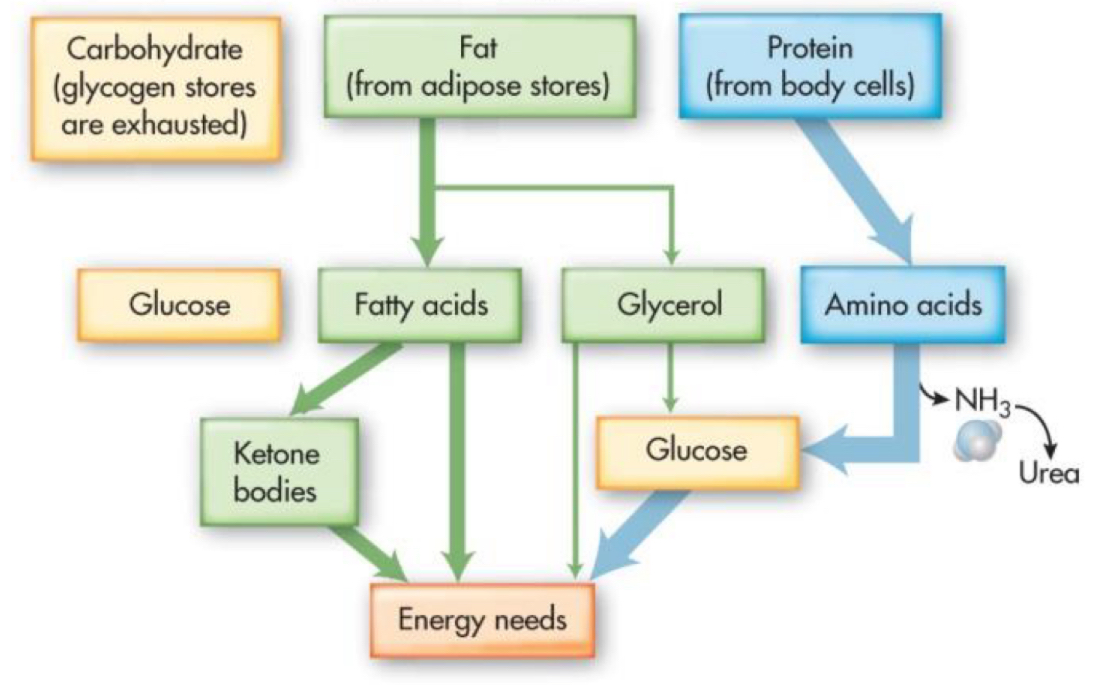
3-5 days after eating (short term fasting)
energy utilization:
protein
fats
5-7 days after eating (long term fasting)
energy utilization:
fats
proteins
phenylketonuria
insufficient phenylalanine hydroxylase activity
cannot convert phenylalanine to tyrosine
form toxic metabolites
management of phenylketonuria
special formula at birth
low phenylalanine diet for life
fruits, vegetables, and breads generally can be eaten
dairy, eggs, meats, nuts, and aspartame must be avoided
galactosemia
cannot convert galactose to glucose
must use soy formula in infancy
throughout life must avoid dairy products, butter, organ meats, and some fruits/vegetables
glycogen storage disease
liver cannot convert glycogen to glucose
leads to:
poor physical health
low blood glucose levels
liver enlargement
management of glycogen storage disease
must consume frequent meals and cornstarch between meals