Organic Chemistry Experiments 1-8
1/105
Earn XP
Description and Tags
Exam 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
106 Terms
how to calculate yield
Convert grams to moles to get Actual Yield
use molar mass to go from g to mol
use density to go from mL ot g
Theoretical Yield
use moles of reactants to determine moles of product
determine limiting reagent:
use mole fraction to find out which reactant gives you the least amount of product
this is your Theoretical Yield
% Yield = Actual Yield of Product / Theoretical Yield * 100
theoretical yield should be higher
wetness or contamination error can mess up % yield
Sig Fig Rules
* and / = lowest number of sig figs
+ and - = fewest decimal places
Fold filter paper to…
increase surface area, making it faster to filter
Water is good for heating because…
it has a high heat capacity
Be careful with magnesium solid because…
it could start a fire
What can smother a fire?
sand bath
Volatile
low boiling point to vaporize
fume hood
What is melting point?
temperature at which phase transitions from solid to liquid
Water Phase Diagram
supercritical fluid next to liquid above gas
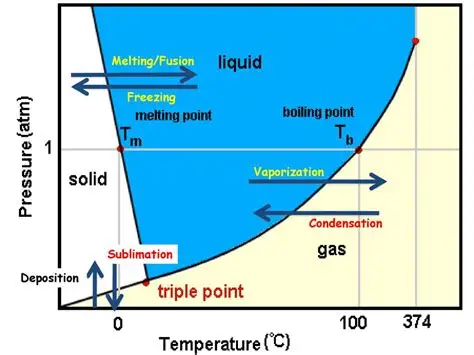
It’s melting not liquification because…
liquification can be any phase → liquid
petrification
gas → solid
What factors affect melting point?
size of molecule/compound
butane (mp = -138 degrees C) vs. octane (mp = -57 degrees C)
larger molecule = more LDFs = stronger IMFs
branching
branched molecules have a lower mp and bp
more stacking if not branched + more LDFs and IMFs = higher mp, higher bp
intermolecular interactions
contamination/mixtures
weakens crystal lattice, causing mp to decrease
Contamination/Mixtures
pure crystal solid has proper orderly crystal lattice which would take a specific heat to melt
contaminant → chaos (increased entropy) → crystal lattice is weaker and will break down with less heat
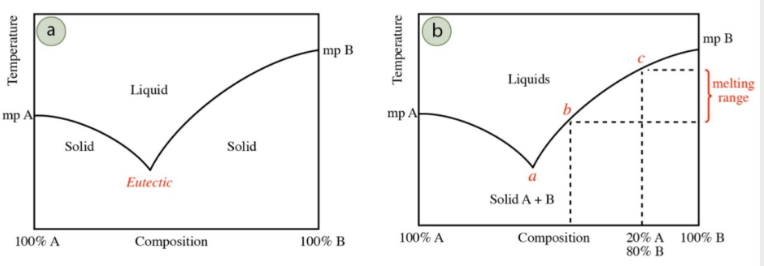
melting point depression
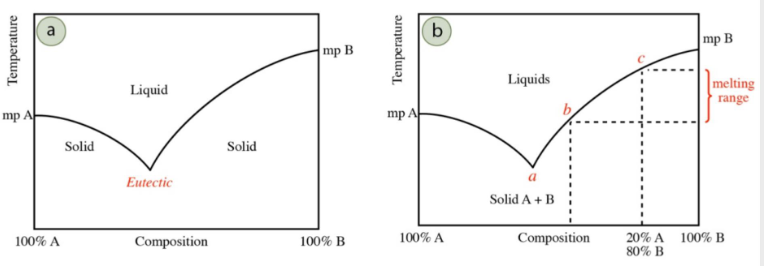
If you have pure A or B and introduce B or A, mp will…
decrease
Eutectic temperature/point
minimum melting point for a mixture
true lowest temperature A & B can reach
as more of another component is introduced, melting point of mixture decreases from original melting points of A and B
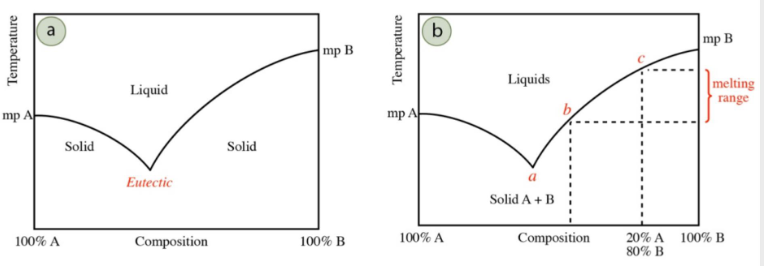
Calibration Curve
gives very precise curve without having to run the experiment
range is between 2 points, mp isn’t always instantaneous
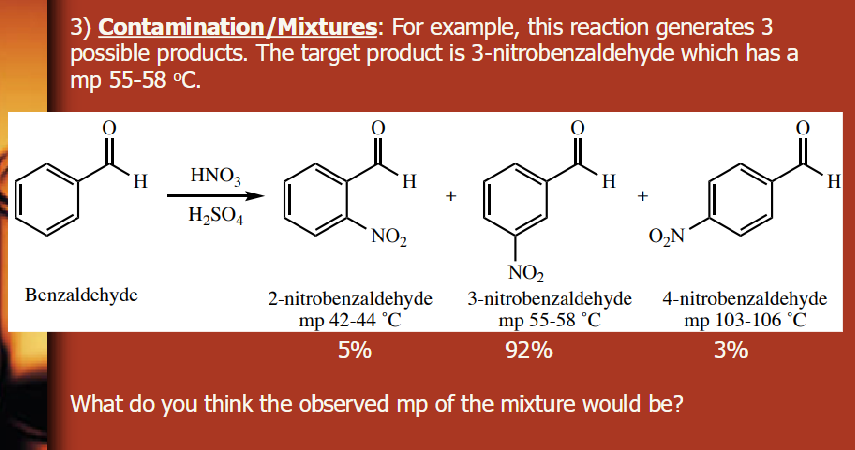
The mp should be lower for the mixture than pure, so less than 55-58 degrees C, but not significantly because it’s 92%.
Pure/Impure/Glucose Sample mp ranges
pure sample: small mp range (± 1-2 degrees C)
impure sample: larger mp range (> ±2 degrees C)
takes longer
pure glucose: mp = 146 degrees C (pure = 145-147)
How to take mp?
need a fine powder and capillary tube
solid grounded down
turn capillary tube upside down and tap into powder
flip over and tap to get into closed part
program the temperature you want and observe sample
first liquid drop = solid → liquid phase has begun = beginning of melting point/range
Recrystallization is a… in which you…
purification technique
isolate a target compound as pure crystals from other components in a crude sample (mixture)
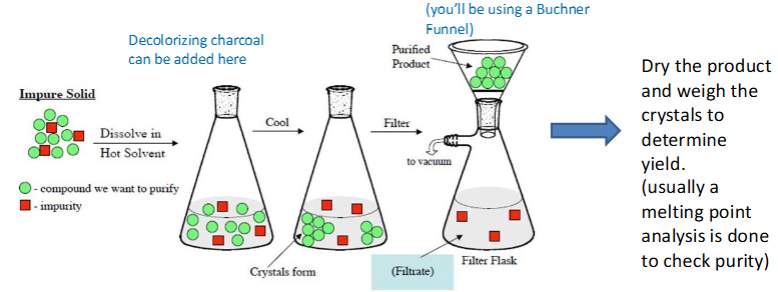
Recrystallization simple overview steps
dissolve solid to release impurities
cool down, pure solid reforms
Recrystallization more in depth steps
crude crystals are dissolved in hot solvent to dissolve unwanted contaminants
during cooling, crystal formation begins (nucleation) and proliferation of crystals (growth) continues
crystals are then collected with filter flask/Buchner Funnel and vacuum, and usually washed with cold solvent
dry product and weigh crystals to determine yield, mp analysis to check purity
Why is it relevant that recrystallization is slow?
it is thermodynamically controlled
slow process = most yield because crystals have time to form crystal lattice
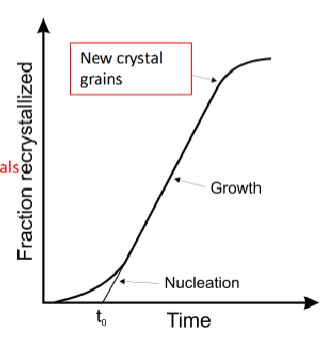
Recrystallization Graph
nucleation: crystal seed is planted
end saturation/crystal formation
Recrystallization Solvent Rules
solute must be relatively insoluble in solvent at room temperature but more soluble in solvent at higher temperature
impurities that are present but be soluble in solvent at room temperature OR insoluble in solvent at high temperature
typically, a solvent with a similar polarity to solute being dissolved will be good
ideal:
unreactive with solutes
inexpensive
low toxicity
solvents with low boiling points (diethyl ether, acetone, low boiling petroleum ether) are highly flammable and can be difficult to work with because they evaporate quickly
good options: high boiling petroleum ether/ligroin, methanol, hexanes, athyl acetate, ethanol, water, toluene
How to determine if Recrystallization was successful
melting point of recrystallized product should be higher than that of the original crude sample
mixtures have lower overall mps
If no crystals form in recrystallization…
glassware may be dirty, try scratching to induce nucleation
too much solvent can cause your crystals to not recrystallize
If you use hot solvent to wash the collected crystals in recrystallization…
some of the crystals will be lost
this means lower yield
always use cold solvent
What are colligative properties?
affected by the amount and number of components in the solution
vapor pressure
boiling-point elevation
freezing-point depression
Freezing Point Depression
lowers freezing point of a liquid when another substance is dissolved in it
deltaT = iKffm
i = van’t Hoff factor (number of components)
Kf = molal freezing point depression constant
m = molality (amounts of each component)
pure water → salt water
freezing point lowers
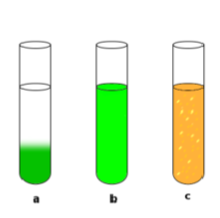
Heterogeneous vs Homogeneous Mixtures
heterogeneous: not uniform in appearance (a and c)
only homogeneous mixtures can be called solutions (b)
solutions = solids dissolved in liquids or liquids dissolved in liquids
Distillation: what is it and why do we perform it?
purification technique
allows us to separate the components of a mixture or purify a liquid based on their different boiling points
Solution mixtures
homogeneous
composed of individual components
we can use distillation if they have different boiling points
ex: acetone (57 degrees C) and water (100 degrees C)
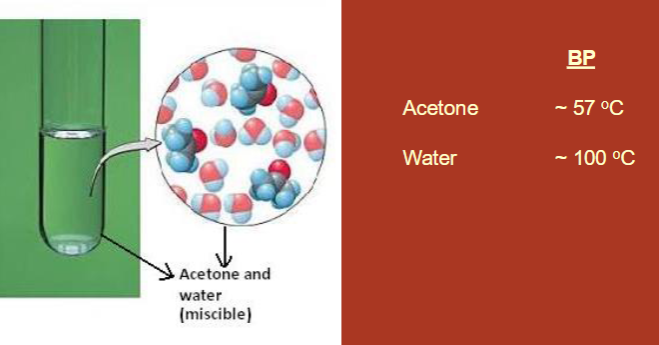
Types of Distillation
simple distillation
fractional distillation
Simple Distillation
used when difference in bp of components of a solution mixture is >70 degrees C
larger gap
usually at least 75 degrees C difference
Dalton’s Law
Fractional Distillation
used when simple distillation of a mixture would be inefficient (difference in bp of mixtures < 70 degrees C)
smaller gap
fractionating column
Dalton’s Law
total vapor pressure of a solution is equal to the sum of the individual vapor pressures of the components
Fractionating Column
allows for multiple equilibrations of the volatile mixture to occur along the height of the column
vapor will rise and condense multiple times in the column
each vaporization/condensation event is called a theoretical plate
more theoretical plates = better separation
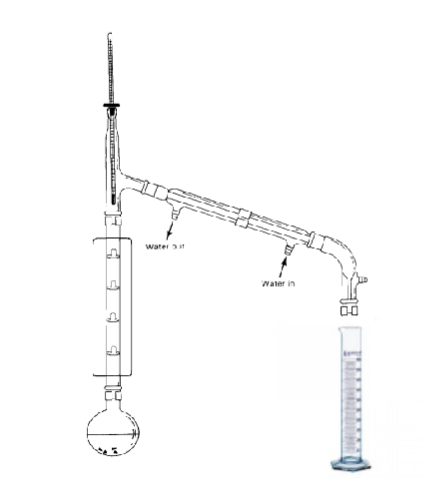
Label:
Bunsen burner
round-bottom flask
fractionating column
thermometer
condenser
Phase Diagram for a general 2-component liquid mixture
middle section = liquid and gas
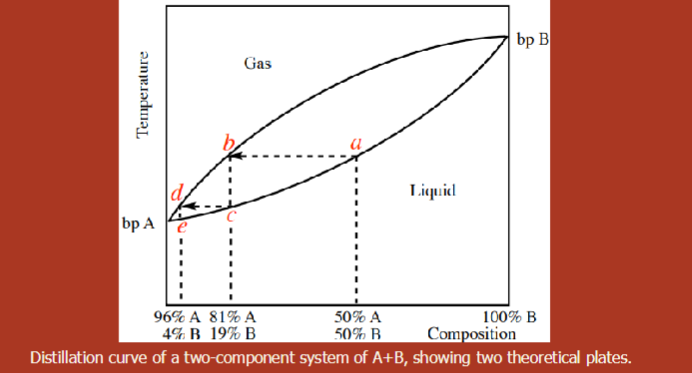
A 50%/50% mixture of two components whose boiling points differ by only 20-30 degrees C would require at least __________ to obtain a distillate with >95% purity.
3 theoretical plates to obtain a distillate with >95% purity
The best chance of obtaining purity via fractional distillation is when…
there is very little impurity to begin with
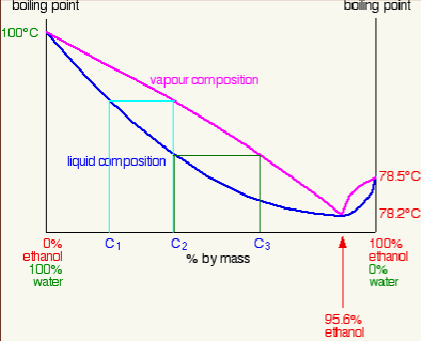
Azeotropes
certain liquid mixtures that are very hard to separate using fractional distillation
methanol and toluene
occurs because vapor pressure above the solution mixture has the same composition as the liquid mixture
two types:
low-boiling
high-boiling
low-boiling azeotrope example
95.6% ethanol in water
will always have a bp of 78.62 when mixed
boil together like one substance with a shared bp
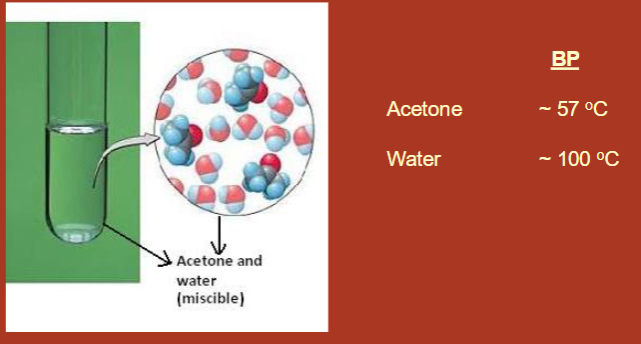
What type of distillation should be used to purify this solution mixture? Which of the two components should be isolated first?
<70 degrees C difference → fractional distillation
acetone should be isolated first because it has a lower boiling point
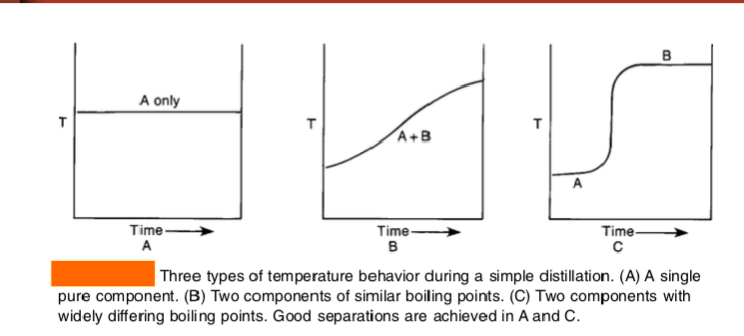
A = single pure component (good)
B = simple distillation used on two components of similar boiling points (not good)
C = fractional distillation used on two components with very different boiling points (good)
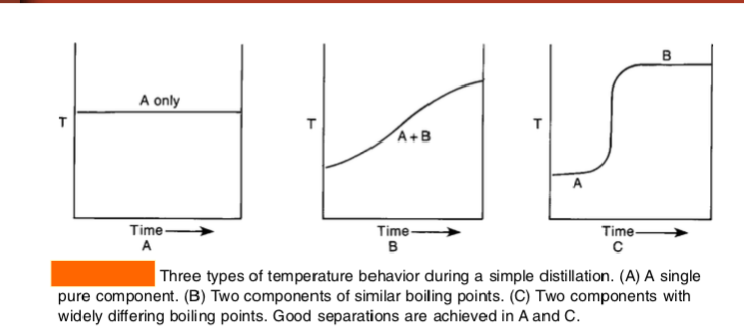
What would a successful distillation look like on a graph of temp vs. fraction collected?
flat temp plateau while one component is boiling off (collecting first substance)
sharp temperature jump to higher plateau, showing next substance starting to distill
c
What fractions are the most pure in distillation?
the fractions closest together in temperature (the plateaus)
Extraction
used when distillation is not possible
some compounds have a really high bp
What factors affect a molecule’s solubility?
IMFs/Vanderwaal’s Forces
H bonding
electrostatic interaction (ionic)
dipole-dipole
H-bonding criteria
H-bond donor (partially + H NOT on carbon), FON
H-acceptor (anything with a lone pair)
Which are soluble in water?
B in its enol form → double bond O becomes single bond OH and double bond C-C
D
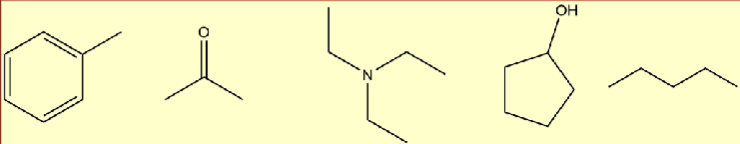
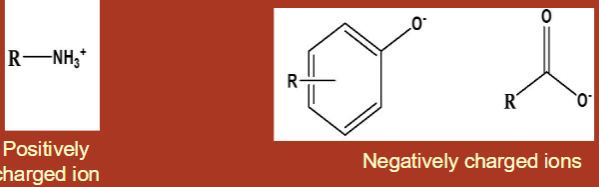
Organic molecules are soluble in water when…
they are either ionic or very polar
positively charged ion or negatively charged ion
water is polar
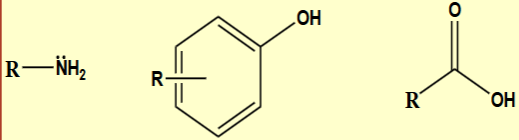
Neutral organic molecules are generally soluble in…
organic solvents
not ions
Ions are __________ in water.
very soluble
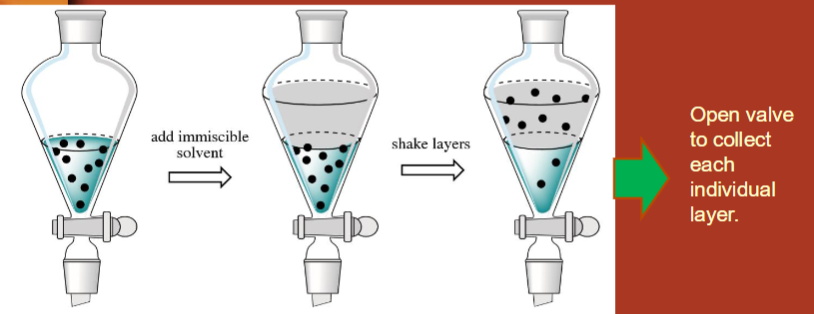
What is liquid-liquid extraction?
compounds in one liquid layer can be extracted into another liquid layer
liquid in separatory funnel → add immiscible solvent → shake layers → add saturated NaCl to deal with emulsion (foamy liquid when you mix too vigorously) → open valve to collect individual layers (in beaker)
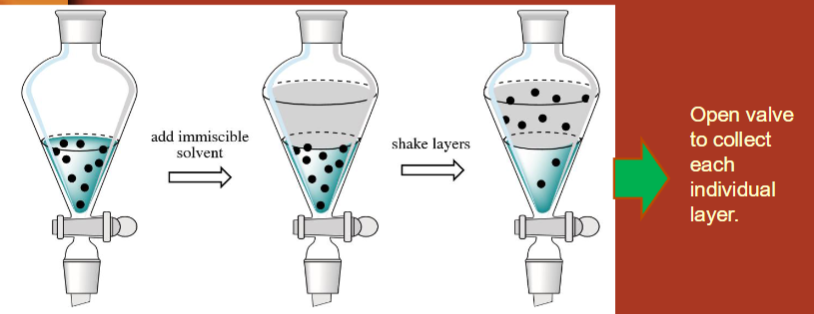
In liquid-liquid extraction, which layer is which?
top layer = organic solvent
bottom layer = aqueous, water is more dense than organic solvents
How do we represent the preference of molecules for organic/aqueous solvents?
Partition Coefficient (K)
we want K > 1 so we can isolate the majority of the target component in the organic layer (organic/top layer is favored)
K = [S]2/[S]1 = organic/aqueous
S2 = Phase 2 = solute in organic phase (top layer)
S1 = Phase 1 = solute in aqueous phase (bottom layer)
K is essentially…
the same regardless of the amount of organic solvent used
volume of organic is unimportant
What determines a successful extraction?
n
the number of times that you do the extraction
more times = better
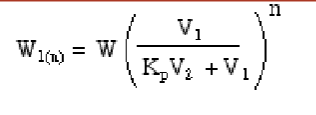
What do variables mean?
W = original weight of compound
W1(n) = weight of compound remaining in Phase 1 after “n” extractions
n = number of extractions
V1 = volume of phase 1
V2 = volume of phase 2 (extracting phase)
Kp = (solubility of W in phase 2) / (solubility of W in phase 1)
The efficiency of extraction is strictly dependent on the…
number of extraction events performed (n)

W = 5.2 g
W1(n) = ?
n = 1
V1 = 60 mL
V2 = 50 mL
K = 8.1
W1(n) = 5.2 (60 / (8.1×50) + 60)^1 = … g leftover
How much was extracted = W - W1(n)
in this instance, should be really high because Kp = 8.1
The conjugate base is much more ______and, therefore, more
soluble in the _________
polar
aqueous phase
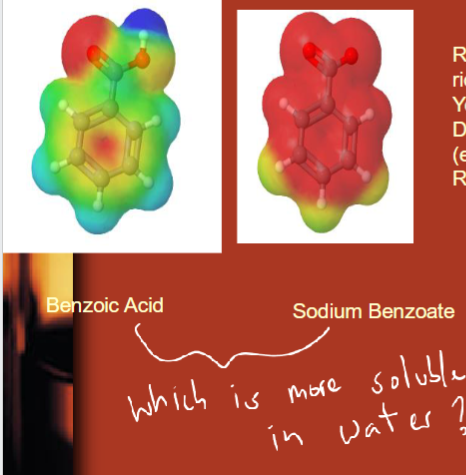
sodium benzoate = conjugate base of benzoic acid
red = partial negative/e- rich
yellow = neutral/np
dark blue = partial positive/e- deficient
red and blue = polar
sodium benzoate is more polar!
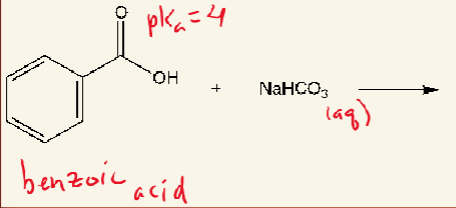
Converting a compound to its conjugate base
very acidic
+ bicarbonate (weak base)
forms carboxylate (just make H negative)
Applications of Extraction
isolation of genomic DNA from human blood
analysis of isolated DNA using agarose gel electrophoresis
extracting vanilla beans in solution of alcohol and water
What is TLC?
detection technique for purity of samples
TLC Phases
competing attractive forces provide an environment for molecules to display their relative polarities
Stationary phase: silica gel plate
polar OH groups
molecules attracted more/less strongly relative to their own polarity
polar molecules grab on tight, nonpolar don’t
Mobile phase
solvent system/liquid
drawn up the plate by capillary action / by solvent
b/c plate is porous, surface area surface tension pulls liquid up
if polar, it will pull polar compounds along more; if np, polar compounds stick to silica gel
What IMFs are happening between polar molecules and silica gel?
hydrogen bonding
dipole-dipole
How far compounds travel up the TLC plate depends on…
how strongly they are attracted to silica gel (how polar they are)
the polarity of the solvent system

Green is most polar because it grabs onto silica gel
Green, Blue, Red
Different solvent systems have different eluting powers, and changing the ratio of the solvents…
changes the results
ethyl acetate:hexane ratio
ethyl acetate is more polar

Left plate: solvent system is less polar (3:1)
Right plate: solvent system is more polar (1:3)
Retention Factor
Rf = distance sample traveled/distance solvent system traveled = d1/d2
values range from 0-1
small Rf = most polar
largest Rf = least polar
same Rf = same compound
2 factors that affect Rf values
polarity of molecules
polarity of solvent
Lipophilicity
comparing non-polar vs polar moieties in molecules
ratio of polar to np (more ch = less polar, lipid-like chain)
What is more polar?
b, less symmetrical, more func. groups
b, more func groups = more polar
Hexane vs. Ethyl Acetate Solvent TLC
hexane = non-polar, sample will not interact strongly
ethyl acetate = polar, sample will move far
Solvent front
point where mobile phase (solvent) had climbed when removing TLC plate (top line)
TLC uses
separating components of mixture
assessing purity of compound (one spot = pure, multiple spots = impure)
identifying components of a mixture, using a cospot
monitoring progress of a reaction (starting material vs. product)
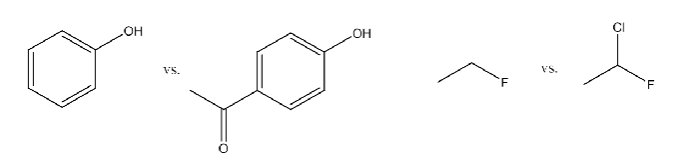
relative stabilities of alkenes
more substituted = more stable
because uses sp²-sp³ bonds for bonding rather than sp³-sp³
sp²-sp³ is stronger which decreases heat of formation
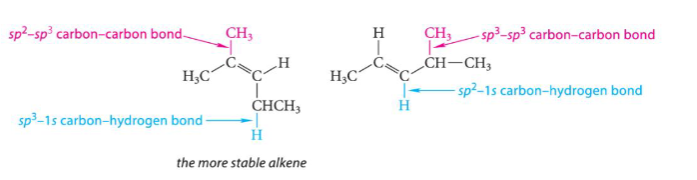
Electrophilic Addition Reactions
carbon-carbon double bond (pi bond) of alkene is broken and X-Y bond of reagent is broken
new C-X and C-Y bonds are formed
pi electrons and lone pair electrons are nucleophile (Lewis base)
nucleophiles attack partial positive or formally positively charged species (H+)
in solvent like Br2 (not H2O)
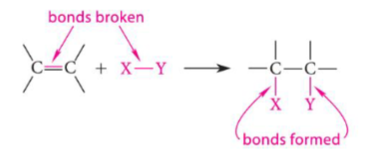
Electrophilic Addition Reactions Reaction Profile
rate-limiting step is highest climb
transition state of highest free energy/activated complex = peak
intermediate present in valley (ie bromonium ion)
exothermic reaction (reactants have higher energy than products)
deltaG rxn = deltaG products - deltaG reactants
deltaG rxn <0 → spontaneous or favorable
Meso Compounds
2+ stereocenters
achiral with at least 2 chiral centers with opposite stereochemistry
have symmetry along center of molecule
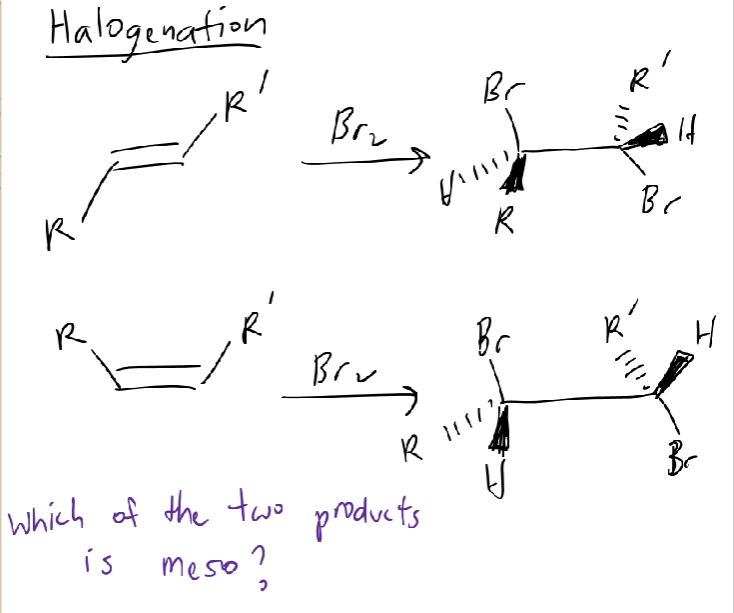
symmetrical
R, S; E or trans
S, S; Z or cis
1 is meso
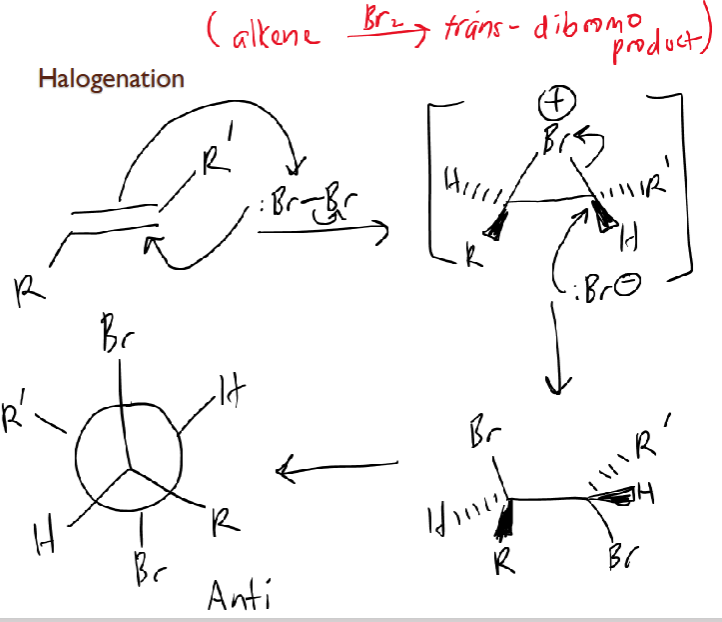
Halogenation
alkene + Br2
Br lp attacks alkene, alkene attacks Br back
forms bromonium ion intermediate: triangle
Br- lp attacks a C, which breaks bond between Br+, forming Br addition on either side adding in anti-addition fashion (antiperiplanar/staggered)
trans-dibromo product
Bromine (Br2) is pretty…
toxic and volatile, so we use pyridinium hydrobromide perbromide instead
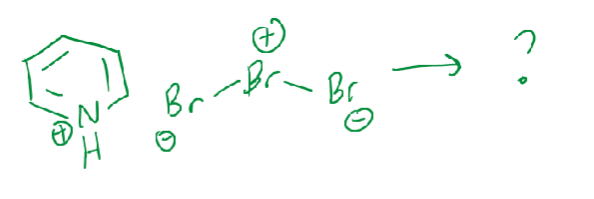
Br2 + N cyclohexene + HBr
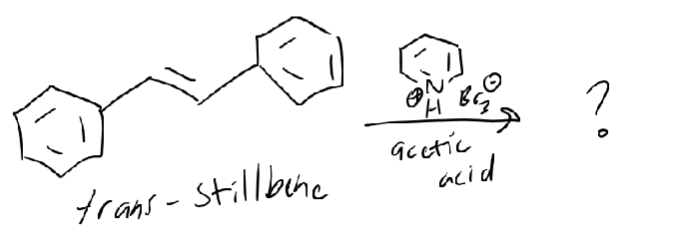
trans-addition of Br
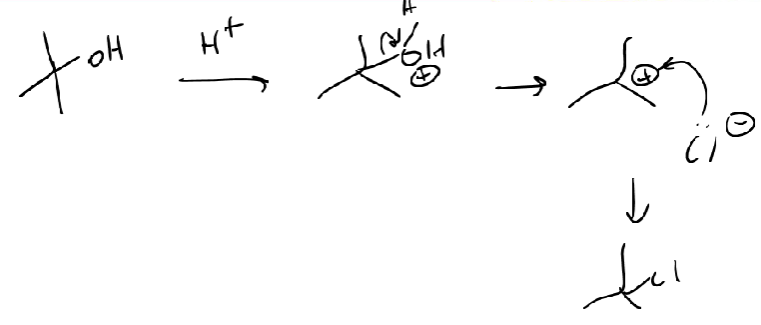
Halogenation Reactions - Alcohol Mechanism
H+ from H-X attacks OH
O becomes positive, H2O leaves
Carbocation forms (strong electrophile)
Now negatively charged X attacks carbocation
not a great nucleophile (wean anion) but good w carbocation
Markovnikov = most substituted (carbocation can move)
Why is hydroxyl (R-OH) such a bad lg?
strong base/nucleophile
= reactive/unstable, will go right back to R group of H+

Why is R-X a better leaving group than R-OH?
X = halogen
X- = weak Lewis base + R+ = conjugate acid → H-X (strong conjugate acid)
lower pKa of <2 (acidic)
halide = weak base = solid
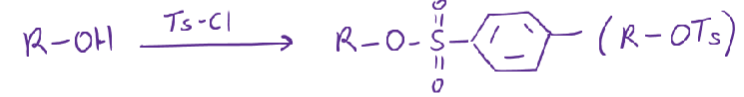
Is the OTs a better LG than OH?
Yes
it’s stable because of resonance (O^-) making it a good LG with a low pKA (acidic)

What mechanism for this reaction?
What kind of alcohol would be best?
What solvent would be best?
SN1 (carbocation)
tertiary → tertiary carbocation
protic solvent (polar) - favors ion formation and stabilizes ions
How can we check success of SN1 alcohol reaction?
use IR
E = hv = hc/wavelength
want to see OH bond 3400-3200 disappear
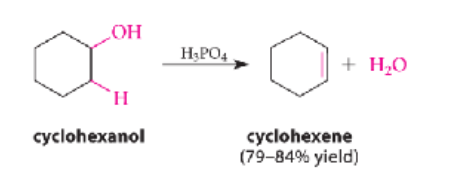
Dehydration of Alcohols makes…
alkenes
strong acids (H2SO4 or H3PO4) catalyze a beta-elimination reaction in which water is lost from a secondary or tertiary alcohol → alkene
carbocation rearrangement
conversion from alcohol to halide could undergo rearrangement
y = 10^x
solve for x
log y = log(10x)
log y = x log10
x = log y / log10 = log y
SN1 vs. E2
SN1: steps, intermediate (carbocation)
E2: concerted, no intermediate