biochemistry exam 3
1/82
Earn XP
Description and Tags
quiz 8 & 9, problem sets 10-13, assignments 10-12
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
polysaccharide that is broken down through
phosphorolysis
glycogen
the type of cell that stores fats
adipocytes
the structure that degrades polyubiquitinated proteins inside the cell
proteasome
the structures that transport lipids in the blood stream
lipoproteins
reduction reaction
gaining an H, electron gain, ex: +3 to 0 net charge
how can you check wether a reaction is reduction or oxidation
Check for Changes: Look at how the oxidation states of elements change from reactants to products.
Decrease = Reduction, Increase = Oxidation: If an element's oxidation state goes down, it's reduction (gaining electrons). If it goes up, it's oxidation (losing electrons).
Think "LEO the Lion Says GER": LEO stands for "Lose Electrons: Oxidation", GER stands for "Gain Electrons: Reduction".
Spot Electron Transfer: If electrons appear on the product side, it's likely a reduction. If they appear on the reactant side, it's likely an oxidation.
Look for Agents: The substance causing oxidation is the oxidizing agent, and the one causing reduction is the reducing agent.
oxidation reaction
losing an H, electron loss, ex: 0 to +2 net charge
why is ADP + Pi more stable than ATP when ATP is hydrolyzed to ADP and a free phosphate, releasing a lot of energy
1) ATP contains 4 negative charges in its phosphate groups, which generate electrostatic repulsion between these charges. Hydrolysis of ATP’s phosphodiester bonds separates or “breaks up” these charges, relieving some of the high-energy electrostatic repulsion between them.
2) The negatively charged phosphate ions released by the hydrolysis of ATP’s phosphodiester bonds are lower in energy (more stable) than the phosphate groups bonded to ATP. This is because the released phosphate ions can share their negative charges (through resonance) with all 4 of the phosphate ion’s oxygens, spreading out the charges. The phosphate ion bound to ATP can only share the negative charges with only 3 of its oxygens, as the 4th oxygen is bound up in a phosphodiester bond. For this reason, the charges are more concentrated (less spread out) in the phosphate bound to ATP, making them higher in energy (less stable) than those in the free phosphate ion.
what cofactor is used in the production of oxaloacetate by pyruvate carboxylase?
Biotin
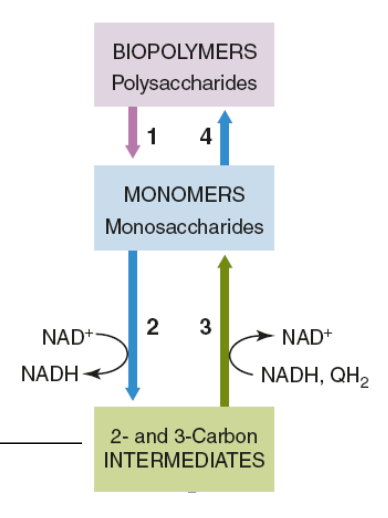
in this figure, which number represents glycolysis?
1
the citric acid cycle is often referred to as a catalytic cycle. which intermediate could be referred to as a catalyst?
oxaloacetate
what is an anaplerotic reaction that is often used in the cell?
conversion of pyruvate to oxaloacetate
which of the following is observed in the mechanism of citrate synthase
A) a histidine residue acts as a base, deprotonating oxaloacetate
B) an aspartic acid residue acts as an acid, protonating oxaloacetate
C) a histidine residue hydrogen bonds with the carbonyl oxygen of oxaloacetate to enable a nucleophilic attack
D) a stabilizing hydrogen bond is formed between an oxyanion (negatively charged oxygen) in the transition state and a hydrogen bond donating histidine amino acid in the active site
E) removal of the CoA is accomplished by transfer to a serine residue, then hydrolysis
D
what is the correct order of product/byproduct released by dehydrogenase
CO2, acetyl-CoA, NADH
what’s the role of PFK-2 in regulating both glycolysis and gluconeogenesis pathways including the names of all the involved enzymes
Phosphofructokinase-2 converts fructose-6-phosphate to fructose-2,6-bisphosphate, which is a potent activator of the phosphofructokinase, which is the primary regulatory step in glycolysis. Therefore, when blood glucose is high, phosphofructokinase-2 will be produced, this enzyme will generate fructose-2,6-bisphosphate and the glycolysis pathway will be activated. On the other hand, the product of phosphofructokinase-2 (fructose-2,6-bisphosphate) deactivates fructose bisphosphatase, a key step in gluconeogenesis. Therefore, when blood glucose is high, the production of phosphofructokinase will also prevent the process of gluconeogenesis from occurring
what are the five cofactors used by the pyruvate dehydrodgenase complex
TPP, lipoamide, coenzyme A, FAD, NAD+
which steps in the TCA cycle are energetically irrecersable
oxaloacetate → citrate, isocitrate → alpha ketoglutarate, alpha ketoglutarate → succinyl coA
why is step one in the TCA cycle irreversible?
oxaloacetate → citrate is irreversible because it is catalyzed by citrate synthase is highly exergonic (ΔG°′ = –31.5 kJ · mol−1). This energy is produced by hydrolyzing the thioester bond of acetyl-CoA.
why are steps 3 and 4 irreversible?
both steps produce carbon dioxide. Because carbon dioxide is a gas, it rapidly diffuses away from the enzyme as soon as it is produced. For this reason, carbon dioxide cannot be recombined with any other products to regenerate the substrate, which makes these reactions irreversible
Explain why most protease enzymes cannot operate in the stomach
The pH in the stomach is ~2, at such strongly acidic pH levels, proteins will become denatured and degraded
explain how, despite the absence of most protease enzymes, digestion of proteins does begin in the stomach
Digestion is the process of breaking up ingested biological polymers (such as polysaccharides and proteins) into smaller units. For the same reason that protease enzymes will become denatured and degraded in the stomach (very acidic conditions), ingested proteins will also begin to degrade under these conditions (be digested). Under acidic aqueous conditions, the amide (peptide) bonds in proteins will hydrolyze, breaking proteins up into smaller units
Describe the meaning of the term “phosphorolysis” in the context of metabolism.
Phosphorolysis is the process cells use to break up glycogen polymers into their individual glucose units. In the process of phosphorolysis, a phosphate ion acts as a nucleophile to cleave the glycosidic bonds of glycogen, cleaving off individual glucose molecules which are released as glucose-1-phosphate
Why does the phosphate group that gets attached to glucose during phosphorolysis need to be removed before glucose can be released into the bloodstream?
As we have discussed, phosphorylated glucose molecules are “trapped” in the cell because they are too polar to diffuse through the lipid bilayer, and because they do not have the correct structure to bind to the glucose transport protein. Therefore, for the glucose molecule to be released into the bloodstream through either of these mechanisms, the phosphate group must be removed.
Explain why ubiquitin is sometimes referred to as the “molecular kiss of death”
intracellular proteins that are no longer needed can be “recycled” by the proteasome, a multi-subunit protein complex that degrades proteins. To ensure that the proteasome does not degrade proteins that are needed, the proteasome will only degrade proteins that have been “tagged” by the cell with the small protein ubiquitin. Once a protein has been “tagged” with ubiquitin, it is only a matter of time before that protein will be degraded by the proteasome. This is the reason why ubiquitin is sometimes referred to as the “molecular kiss of death”.
We have discussed that ATP hydrolysis to ADP and a free phosphate releases a significant amount of energy. Explain why the products of this reaction (ADP + Pi) are more stable than the substrate (ATP)
There are 2 primary reasons why the products of ATP hydrolysis are more stable than ATP itself.
1) ATP contains 4 negative charges in its phosphate groups, which generate electrostatic repulsion between these charges. Hydrolysis of ATP’s phosphodiester bonds separates or “breaks up” these charges, relieving some of the high-energy electrostatic repulsion between them. 2) The negatively charged phosphate ions released by the hydrolysis of ATP’s phosphodiester bonds are lower in energy (more stable) than the phosphate groups bonded to ATP. This is because the released phosphate ions can share their negative charges (through resonance) with all 4 of the phosphate ion’s oxygens, spreading out the charges. The phosphate ion bound to ATP can only share the negative charges with only 3 of its oxygens, as the 4th oxygen is bound up in a phosphodiester bond. For this reason, the charges are more concentrated (less spread out) in the phosphate bound to ATP, making them higher in energy (less stable) than those in the free phosphate ion.
Although ATP is commonly referred to as the “energy currency” of the cell, other biological compounds are also used by the cell in a similar manner to drive energetically unfavorable reactions forward. Provide two examples of other biological molecules that can act as energy sources for the cell and describe the functional groups that store energy in these molecules.
acetyl coA, phosphocreatine
which type of reaction is step one (hexokinase)
phosphate transfer
which type of reaction is step two (phosphoglucose isomerase)
isomerization
which type of reaction is step three (PFK)
phosphate transfer
which type of reaction is step four (aldolase)
carbon-carbon cleavage
which type of reaction is step five (triose phosphate isomerase)
isomerization
which type of reaction is step six (GAP dehydrogenase)
oxidation-reduction/phosphate transfer
which type of reaction is step seven (phosphoglycerate kinase)
phosphate transfer
which type of reaction is step eight (phosphoglycerate mutase)
isomerization/phosphate transfer
which type of reaction is step nine (enolase)
dehydration
which type of reaction is step ten (pyruvate kinase)
isomerization/phosphate transfer
Which of the reactions in the glycolysis pathway are irreversible?
Steps 1 (catalyzed by hexokinase), 3 (catalyzed by phosphofructokinase) and 10 (catalyzed by pyruvate kinase)
What is the physiological significance of the irreversible reactions?
these steps are theoretically good potential control points for the flow of metabolites through the glycolysis pathway. This large free energy change also makes these steps energetically irreversible, which means that the gluconeogenesis pathway requires unique enzymes to “get around” these energetically irreversible steps.
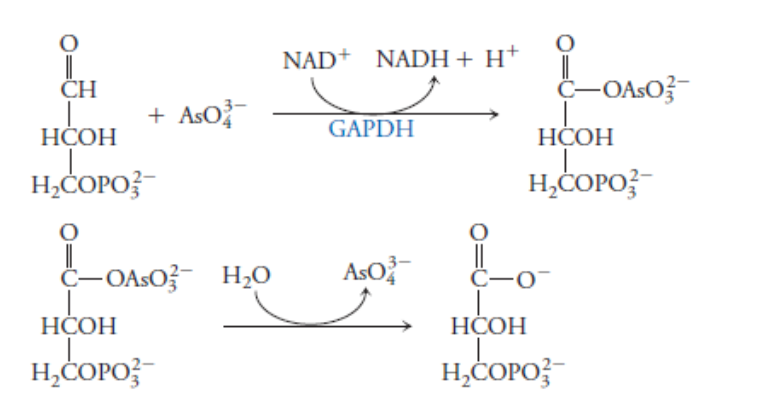
Arsenate (AsO4-3) is a very strong poison because it acts as a phosphate analog and can replace phosphate in the GAPDH reaction (below). The product of this reaction is 1-arseno-3-phosphoglycerate. It is unstable and spontaneously hydrolyzes to form 3-phosphoglycerate (below). What is the effect of arsenate on the overall glycolysis process?
GAP dehydrogenase catalyzes the 6th step in the glycolysis pathway, which under normal circumstances is the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate (below). The product of this reaction (1,3-bisphosphoglycerate) is then converted in step 7 to 3-phosphoglycerate (bottom). In the presence of arsenate, the arsenate substitutes for a phosphate in the reaction catalyzed by GAP dehydrogenase, producing 1-arseno-3-phosphoglycerate, which then spontaneously hydrolyzes to 3-phosphyglycerate (above), essentially skipping step 7. This means that the presence of arsenate prevents the acyl phosphate functional group of 1,3-bisphosphoglycerate from forming. It is the energy released by the cleavage of this acyl phosphate group in step 7 that drives the synthesis of ATP. By preventing the formation of 1,3-bisphosphoglycerate, arsenate bypasses step 7 of glycolysis, which is one of the energy-producing steps. This has the effect of making glycolysis an overall energy-neutral process, rather than an energy-producing process.
Steps 2 (catalyzed by phosphoglucose isomerase) and 6 (catalyzed by GAP dehydrogenase) are examples of reactions in the glycolysis pathway that occur with positive (unfavorable) standard free energy changes. Despite this, both of these reactions occur in the cell with negative (favorable) actual free energy changes. Describe how these reactions are able to occur with negative actual free energy changes (the answer will be different for the two reactions).
The actual free energy change of a reaction depends not only on the standard free energy change, but also on the temperature of the reaction and the concentration of the products and the reactants. If the concentration of the reactants is low for a given reaction, there will be an energetic driving force for the establishment of equilibrium, meaning the production of the products will become more energetically favorable. Therefore, in the case of step 2 in glycolysis, the reaction is able to occur with a negative actual free energy change because the concentration of the reactants is kept very low at all times.
Coupling an energetically unfavorable reaction with an energetically favorable reaction is another way to make a reaction favorable. In the case of step 6, the cell ensures that this reaction occurs essentially in concert with step 7, which is highly energetically favorable. Therefore, the “coupled” reactions in step 6 and step 7 occur with an overall negative free energy change.
Pyruvate is the end product of glycolysis, but that does not mean pyruvate is not useful to the cell. Describe 3 ways that pyruvate can be utilized by cells.
1) it can be converted to acetyl-CoA by pyruvate dehydrogenase, which can then enter the citric acid cycle for additional energy production (acetyl-CoA is also used in fatty acid synthesis);
2) it can be converted to lactate or ethanol (depending on the organism) to regenerate NAD+ cofactors required for glycolysis;
3) it can be converted to oxaloacetate by pyruvate carboxylase which is used to synthesize amino acids or for gluconeogenesis.
Pyruvate is the end product of glycolysis, but that does not mean pyruvate is not useful to the cell. Describe 3 ways that pyruvate can be utilized by cells.
1) it can be converted to acetyl-CoA by pyruvate dehydrogenase, which can then enter the citric acid cycle for additional energy production (acetyl-CoA is also used in fatty acid synthesis);
2) it can be converted to lactate or ethanol (depending on the organism) to regenerate NAD+ cofactors required for glycolysis;
3) it can be converted to oxaloacetate by pyruvate carboxylase which is used to synthesize amino acids or for gluconeogenesis.
Organisms such as yeast growing under anaerobic conditions (no oxygen) can convert pyruvate to alcohol (ethanol) in a process called fermentation that is well-known to craft beer enthusiasts. Instead of being converted to lactate, pyruvate is converted to ethanol in a two-step reaction. Why is the second step of this process essential to the yeast cell?
Yeast performs fermentation to regenerate NAD+ cofactors which are consumed by glycolysis. Under anaerobic conditions, yeast uses glycolysis to produce energy. That glycolysis process uses up oxidized NAD+ cofactors and produces reduced NADH cofactors. For the yeast cells to be able to continue to use glycolysis to produce energy, the NAD+ cofactors used in glycolysis must be regenerated. The second step in the fermentation process reduces acetaldehyde to ethanol, while simultaneously oxidizing NADH to NAD+. This process, catalyzed by alcohol dehydrogenase, regenerates the NAD+ cofactors required for glycolysis, allowing the yeast cells to continue to use glycolysis to produce energy
Many traditional baking and brewing methods involve adding amylase (in other words spit!) to bread dough or to the brewing vessels prior to the fermentation process. What is the value of this process?
Fermentation works because glucose is first broken down to pyruvate, which is then converted to ethanol and carbon dioxide in the fermentation process. Because the sugar source for most brewing and baking processes is not glucose, but instead is starch, the starch must first be broken down to glucose before glycolysis and fermentation can occur. Saliva contains amylase, which helps speed up the process of breaking down starch to glucose, and therefore speeds up the fermentation process.
insulin is one of the major hormones that regulates gluconeogenesis. Insulin acts by decreasing the transcription of some of the genes coding for certain enzymes involved in gluconeogenesis. For which genes would you expect insulin to suppress transcription? Justify your choice(s).
There are four enzymes which are specific to the gluconeogenesis pathway. These are the enzymes which allow the gluconeogenesis pathway to “get around” the energetically irreversible steps in the glycolysis pathway. The four enzymes are glucose-6-phosphatase, fructose bisphosphatase, phosphoenolpyruvate carboxykinase, and pyruvate carboxylase. These are the genes that would be expected to be suppressed by insulin.
The role of the pyruvate dehydrogenase complex is to convert pyruvate to acetyl-CoA, however in this 5-step process the product (acetyl-CoA) is released in step 3. What are the roles of step 4 and step 5?
Steps 4 and 5 are important for cofactor regeneration. In step 4, the lipoamide cofactor is oxidized by an FAD disulfide cofactor, restoring the lipoamide cofactor to the state required for additional reaction cycles. This FAD disulfide must then be reoxidized in step 5, which is performed by an NAD+ cofactor
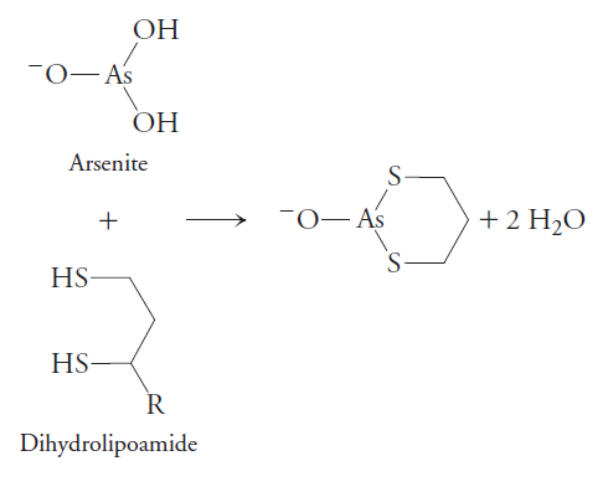
Arsenite is toxic in part because it binds to sulfhydryl compounds such as lipoamide (below). What effect would the presence of arsenite have on the citric acid cycle?
In the presence of arsenite, the reduced form of lipoamide will form the arsenite conjugate shown above. This will prevent the lipoamide cofactor of the pyruvate dehydrogenase complex from being oxidized to its disulfide form by the E3 enzyme. This oxidation is necessary to regenerate the lipoamide cofactor after performing one catalytic cycle. Because the lipoamide cofactor cannot be regenerated, the enzyme will not longer be able to convert pyruvate to acetyl-CoA. This will halt the citric acid cycle because there will be no acetyl-CoA available to oxidize.
The citric acid cycle is often referred to as a “catalytic cycle”. What defining feature of a “catalyst” does the citric acid cycle demonstrate? Which citric acid cycle intermediate could be referred to as a “catalyst”?
The defining feature of a “catalyst” that the citric acid cycle demonstrates is that a catalyst increases the rate of a reaction without being consumed in that process. In the first step of the citric acid cycle, oxaloacetate reacts with acetyl-CoA to generate citrate. In the final step of the citric acid cycle, oxaloacetate is produced from malate. Therefore, it can be said that oxaloacetate is the intermediate that increases the rate of a reaction but is not consumed in the process
Site-directed mutagenesis techniques were used to synthesize a mutant citrate synthase enzyme in which the active-site histidine was converted to an alanine. Why did the mutant citrate synthase enzyme exhibit decreased catalytic activity?
In the mechanism of citrate synthase, an active site histidine residue acts through acid-base catalysis to stabilize the enolate transition state (oxyanion). This transition state stabilization lowers the transition state energy, which lowers the activation barrier (activation energy) for this reaction, allowing more substrate molecules to be converted to product per unit time. When this active site histidine is mutated to alanine, the new alanine residue will not be able to stabilize the enolate transition state because it does not have any hydrogen bond donors. Because the enzyme will no longer be able to provide this transition state stabilization, the catalytic activity decreases.
Does the citrate synthase mechanism employ covalent catalysis, acid-base catalysis, metal-ion catalysis, or some combination of these? Explain.
The citrate synthase mechanism does not demonstrate covalent catalysis, because no covalent bond is formed between the substrate and the enzyme.
The citrate synthase mechanism does not demonstrate metal ion catalysis, because there is no metal ion present in the active site of the enzyme.
The citrate synthase mechanism does demonstrate acid-base catalysis. In the mechanism, a stabilizing hydrogen bond is formed between an oxyanion (negatively charged oxygen) in the transition state and a hydrogen bond donating histidine amino acid in the active site. This decreases the energy of the transition state, lowering the activation energy, and therefore speeding up the reaction. This is just one example of acid-base catalysis occurring in this mechanism (there are others as well)
The crystal structure of isocitrate dehydrogenase shows that there is a cluster of highly conserved amino acids in the substrate binding pocket/active site—three arginines, a tyrosine, and a lysine. Why are these residues conserved? (Predict their role(s)
Arginine and lysine are positively charged amino acids (at physiological pH), and tyrosine can act as a hydrogen bond donor. The substrate in this reaction (isocitrate) has three negative charges, therefore, these amino acids may form hydrogen bonds or ionic associations to help the substrate bind to the active site.
Furthermore, the transition state of this reaction possesses an oxyanion (negatively charged oxygen).
Therefore, these amino acids may form hydrogen bonds or ionic associations with this transition state, helping to spread out its charge, stabilizing the transition state, lowering the activation energy and speeding up the reaction
Malonate is a competitive inhibitor of succinate dehydrogenase. What citric acid cycle intermediates accumulate in the presence of malonate?
Succinate dehydrogenase catalyzes the conversion of succinate to malate (step 6 of the citric acid cycle). In the presence of malonate, this process will be inhibited, and the concentration succinate will increase. We also know that step 5 of the citric acid cycle (the conversion of succinyl-CoA to succinate by succinyl-CoA synthetase) is a near-equilibrium reaction which means it can proceed in either direction depending on the concentration of substrates and products. As the concentration of succinate rises due to the inhibition of succinate dehydrogenase, step 5 will begin to proceed in reverse, causing the concentration of succinyl-CoA to increase. Step 4 is irreversible, and cannot proceed in reverse. Therefore the inhibition of succinate dehydrogenase would cause an increase in succinate and succinyl-CoA concentrations.
Why is the reaction catalyzed by pyruvate carboxylase (conversion of pyruvate to oxaloacetate) the most important anaplerotic reaction of the citric acid cycle? Why is the activation of pyruvate carboxylase by acetyl-CoA a good regulatory strategy?
Oxaloacetate is an important metabolite in the cell as it is used for gluconeogenesis and amino acid synthesis as well as in the citric acid cycle. For this reason, the oxaloacetate produced by the citric acid cycle is constantly being siphoned off for other cellular processes and it must be constantly replaced by the cell for the citric acid cycle to continue. Furthermore, oxaloacetate acts as the “catalyst” in the citric acid cycle, reacting with acetyl-CoA in the first step in the cycle. If there is no oxaloacetate present to react with acetyl-CoA, the rest of the citric acid cycle cannot proceed.
The activation of pyruvate carboxylase by acetyl-CoA is a good regulatory strategy because a high concentration of acetyl-CoA is a signal that the citric acid cycle is not processing acetyl-CoA as quickly as it is being produced. In this situation, the cell will want to increase the catalytic activity of the citric acid cycle. By activating pyruvate decarboxylase, the acetyl-CoA will cause more oxaloacetate to be produced, which will increase the overall rate at which the citric acid cycle can operate.
Oxygen does not appear as a reactant in any of the citric acid cycle reactions, yet it is essential for the proper functioning of the cycle. Explain why
The citric acid cycle oxidizes acetyl-CoA to carbon dioxide, but simultaneously reduces many NAD+, FAD and ubiquinone cofactors. If those cofactors are not oxidized back to their original state, the citric acid cycle will rapidly run out of oxidized cofactors and will not be able to continue. Oxygen acts as the final electron acceptor through a process called oxidation phosphorylation, which oxidizes the reduced cofactors produced by the citric acid cycle. Therefore, oxygen is required to maintain the required concentration of oxidized cofactors for the citric acid cycle to operate.
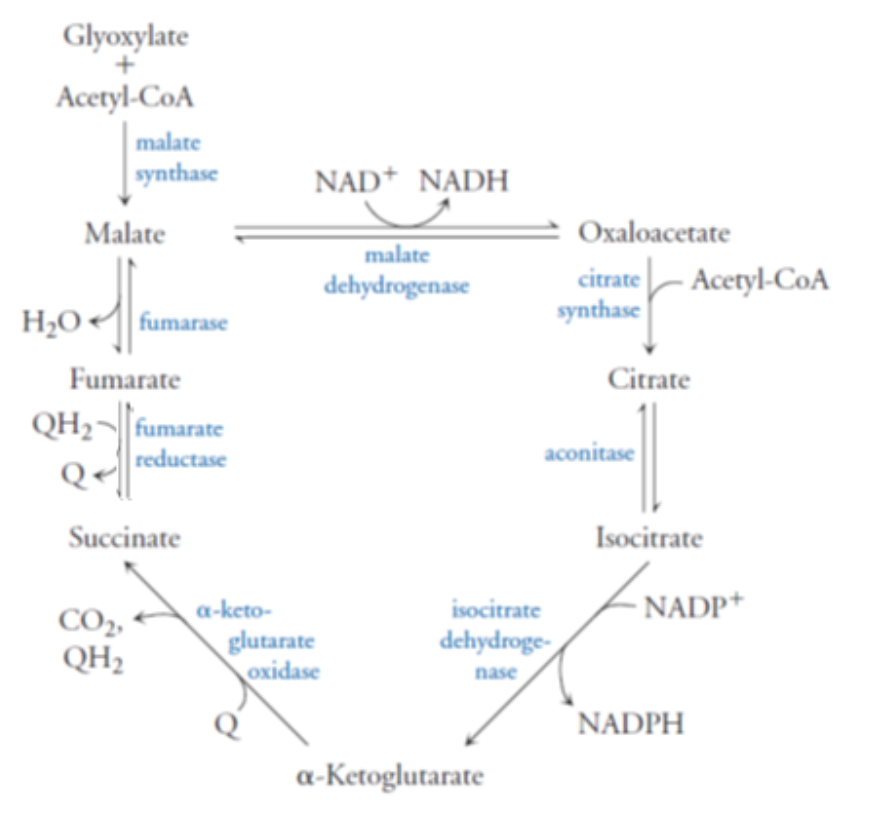
Helicobacter pylori is a bacterium that colonizes the upper gastrointestinal tract in humans, causing chronic gastritis, ulcers, and possibly gastric cancer. Knowledge of the metabolism of this organism will be helpful in the development of effective drug therapies to treat these diseases. H. pylori uses an alternate citric acid “cycle” to produce biosynthetic intermediates instead of metabolic energy (below).
a) What enzymes might serve to regulate the citric acid cycle in H. pylori? Explain your choice(s).
The enzymes that are most likely to be the points at which this organism regulates the citric acid cycle are those that catalyze irreversible reactions. Those irreversible reactions in this pathway are catalyzed by citrate synthase, isocitrate dehydrogenase and α-ketoglutarate oxidase, therefore these enzymes are the likely control points for this pathway.
Helicobacter pylori is a bacterium that colonizes the upper gastrointestinal tract in humans, causing chronic gastritis, ulcers, and possibly gastric cancer. Knowledge of the metabolism of this organism will be helpful in the development of effective drug therapies to treat these diseases. H. pylori uses an alternate citric acid “cycle” to produce biosynthetic intermediates instead of metabolic energy (below)
What enzymes might make good drug targets for persons suffering from gastritis, ulcers, or gastric cancer?
A good drug would target the enzymes specific to the pathogenic organism, while not affecting the enzymes that the pathogen has in common with the host (human). In this case, three enzymes are unique to the H. pylori citric acid cycle that are found in the human citric acid cycle. These enzymes are a-ketoglutarate oxidase, fumarate reductase, and malate synthase. Therefore, it is these three enzymes that would make good drug targets.
Complex I is a flavoprotein (contains an FMN prosthetic group). Why is the FMN cofactor ideal for the electron transfer performed by Complex I?
the role of Complex I is to pass electrons on from NADH to ubiquinol. In order to do this, Complex I facilitates the transfer of electrons from NADH to FMN, and then from FMN to ubiquinone (2 redox reactions). In order for any redox reaction to proceed spontaneously, the change in reduction potential for that reaction must be favorable. The reduction potential of flavin cofactors is intermediate between the reduction potential of NADH and the reduction potential of ubiquinone. This means that the reduction of FMN by NADH will be a favorable process, and the reduction of ubiquinone by FMNH2 will be a favorable process, making FMN an ideal cofactor for Complex I.
Cyanide (CN-) is known to block electron transport in Complex IV by binding to Fe+2 in the Fe-Cu binuclear center.
What happens to oxygen consumption when cyanide is added to respiring mitochondria?
Oxygen acts as the final electron acceptor for the mitochondrial electron transport chain. When cyanide is present, electrons cannot flow through Complex IV to be accepted by oxygen, therefore oxygen consumption will decrease drastically.
Cyanide (CN-) is known to block electron transport in Complex IV by binding to Fe+2 in the Fe-Cu binuclear center.
How would the presence of cyanide effect the concentration of NADH in the mitochondrial matrix? How would this affect the citric acid cycle?
When electron transport through Complex IV is blocked, the entire electron transport chain gets backed up.
Cytochrome c cannot pass its electrons onto Complex IV, therefore the concentration of reduced cytochrome c increases. With no oxidized cytochrome c available, Complex III cannot pass its electrons on to cytochrome c. Because Complex III cannot pass electrons to cytochrome c, it cannot accept electrons from ubiquinol, and the concentration of ubiquinol increases. Because there is no ubiquinone available (oxidized ubiquinol), Complex I cannot pass electrons from NADH to ubiquinone. Therefore, the overall NADH concentration increases significantly. Since NADH acts as a negative allosteric modulator of the citric acid cycle (steps 1, 3, and 4), the rate of the citric acid would decrease significantly
Explain why oxidative phosphorylation cannot occur in mitochondrial preparations to which detergents have been added
As we have discussed in the past, detergents are amphipathic compounds which can disrupt membranes by intercalating into the membrane. The production of ATP in the mitochondria (oxidative phosphorylation) relies on the electrochemical gradient that develops across the inner mitochondrial membrane. When detergents are added, the inner mitochondrial membrane will be disrupted, and therefore the electrochemical gradient will not be able to develop. This will prevent ATP synthesis as there will not be any energetic driving force pushing protons through ATP synthase.
When a culture of yeast grown under anaerobic conditions is exposed to oxygen, a dramatic decrease in glucose consumption is observed (this is called the Pasteur effect). Explain the Pasteur effect.
Yeast cultures growing under anaerobic conditions must use glycolysis to produce ATP. The process of glycolysis produces a relatively small amount of ATP, and the yeast cells must convert the pyruvate products to ethanol to regenerate the NAD+ cofactors required for glycolysis. When the yeast cells are exposed to oxygen, they can immediately begin to use oxidative phosphorylation to produce ATP. Oxidative phosphorylation produces 36 ATP molecules for every glucose molecule metabolized. For this reason, the yeast cells must metabolize many more molecules of glucose (through glycolysis) when growing under anaerobic to produce the same amount of ATP that can be produced when oxygen is present. Therefore, when these yeast cells are exposed to oxygen, they can significantly decrease their glucose consumption while producing the same amount of ATP.
8. Describe how ATP synthase uses the force of a proton moving from the intermembrane space to the matrix to synthesize ATP. Be sure to (at least) describe the role of ATP synthase subunits a, c, ɣ, and β.
The electrochemical gradient (proton motive force), drives a proton from the intermembrane space into the a-subunit of ATP synthase. The proton travels through the a-subunit and into one of the c-subunits which are arranged in a ring. The force of this proton movement causes the ring of c-subunits to rotate, releasing another proton which travels through the a-subunit and back into the mitochondrial matrix. The rotation of the ring of c-subunits causes the simultaneous rotation of the ɣ-subunit, which projects into the mitochondrial matrix. The ɣ-subunit interacts with 3 dimers of α- and β-subunits. As the ɣ-subunit rotates, it causes conformational changes in the α/β-subunit dimers, and it is these conformational changes that drive ATP synthesis. In the loose conformation of these dimeric units, ADP and a phosphate ion can bind, in the tight conformation the ADP and phosphate ion are forced into tight contact, resulting in the synthesis of ATP, and in the open conformation the new ATP molecule is released.
Dinitrophenol (DNP, below) is a lipid-soluble compound with a pKa ~7. When dinitrophenol enters the mitochondria, it can decouple electron transport from ATP synthesis.
a) Explain how dinitrophenol decouples electron transport from ATP synthesis (Hint: think about the relative pH of the mitochondrial matrix and the intermembrane space)
Because of DNP’s pKa, it will become protonated under mildly acidic conditions, and deprotonated under mildly basic conditions. In the mitochondria while oxidative phosphorylation is active, the intermembrane space will be more acidic (higher [H+]) than the matrix. Therefore, when DNP is present in the mitochondria, it can pick up protons (become protonated) in the intermembrane space. Because this compound is lipid-soluble, it can then travel through the inner mitochondrial membrane, carrying the proton it picked up in the intermembrane space, into the matrix. The pH in the matrix will be much higher, and therefore DNP will become deprotonated (release its proton). DNP can then travel back through the inner membrane and repeat this cycle. In this way, DNP can act as a proton shuttle, carrying protons from the intermembrane space and into the matrix. This will destroy the electrochemical gradient because although the electron transport chain will continue pumping protons from the matrix into the intermembrane space, DNP will counteract that action by acting as a proton transporter in the opposite direction. This will “decouple” the electron transport chain from ATP synthesis because although the electron transport chain is still working, it cannot develop the electrochemical gradient required for ATP synthesis.
Dinitrophenol (DNP, below) is a lipid-soluble compound with a pKa ~7. When dinitrophenol enters the mitochondria, it can decouple electron transport from ATP synthesis.
In the 1920s, dinitrophenol was introduced as a diet pill, but was discontinued because the side effects were often fatal. Why would dinitrophenol be an effective diet aid?
Dinitrophenol would be an effective diet aid because it decouples the electron transport chain from ATP synthesis. This means that the body will continue to metabolize glucose and other energy sources which produce the cofactors utilized by the electron transport chain (burn up sugars, fats, etc.). Despite this, the body will not be able to convert those energy sources into ATP synthesis through oxidative phosphorylation, because the decoupling action of DNP prevents the formation of an electrochemical gradient. Therefore, in the presence of DNP the body will increase its rate of metabolism (burning up sugars, fats, etc.) to compensate for this decrease in ATP production.
Which of the following is mobilized for energy use by a phosphorolysis reaction, not a hydrolysis?
glycogen
protein
triacylglycerols
polynucleotides
cholesterol esters
glycogen
which is more highly oxidized that acetaldehyde? ethane, ethanol, acetic acid, ethylene, ethelyne glycol
acetic acid
6) Which of the following factors contributes to the highly exergonic nature of ATP hydrolysis?
removal of phosphate from the cytoplasm
addition of water to the hydrophilic ATP molecule
decrease in negative-ion repulsion in ATP
low energy of activation for the hydrolysis
none of the above
decrease in negative ion repulsion in ATP
whats the net products of glycolysis from one molecule of glucose
2 pyruvate, 2 NADH, 2 ATP
in glycolysis, whats the net gain of ATP during the energy investment phase
-2
in glycolysis what is the net gain of ATP during the energy payoff phase
4
in the reaction catalyzed by aldolase, the bond broken is between carbons 3 and 4 of the substrate. What functional groups are present on these two carbons (C3 and C4) in the products?
c3: alcohol, c4: aldehyde
Experimental evidence indicates that glyceraldehyde-3-phosphate dehydrogenase contains a critical _____ (amino acid) residue in its active site, as shown by its inactivation by iodoacetamide
cys
What coenzyme is required for the conversion of pyruvate to oxaloacetate?
biotin
fructose-2,6-bisphosphate is an activator of _____
PFK
fructose-2,6-bisphosphate is an inhibitor of _____
fructose bisphosphate
which pyruvate dehydrogenase enzyme is paired with TPP
E1
which pyruvate dehydrogenase enzyme is paired with lipoamide
E2
which pyruvate dehydrogenase enzyme is paired with FAD and NAD+
E3
Which coenzyme is directly responsible for the oxidation of the hydroxyethyl group to the acetyl group?
lipoamide
what is the mechanism of citrate synthase
an aspartic acid residue acts as a base, deprotonating acetyl-CoA
How does the reaction catalyzed by malate dehydrogenase proceed despite a
∆G°′ of 29.7 kJ/mol
concentrations of oxaloacetate are kept very low by rapid use in the subsequent step
whats an anapleurotic reaction often used in the cell
conversion of pyruvate to oxaloacetate