4.4- Atomic Structure and Radiation
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
What was the first model of the atom
Hard Spheres which can't be broken down any further
John Dalton
Second Model of the atom
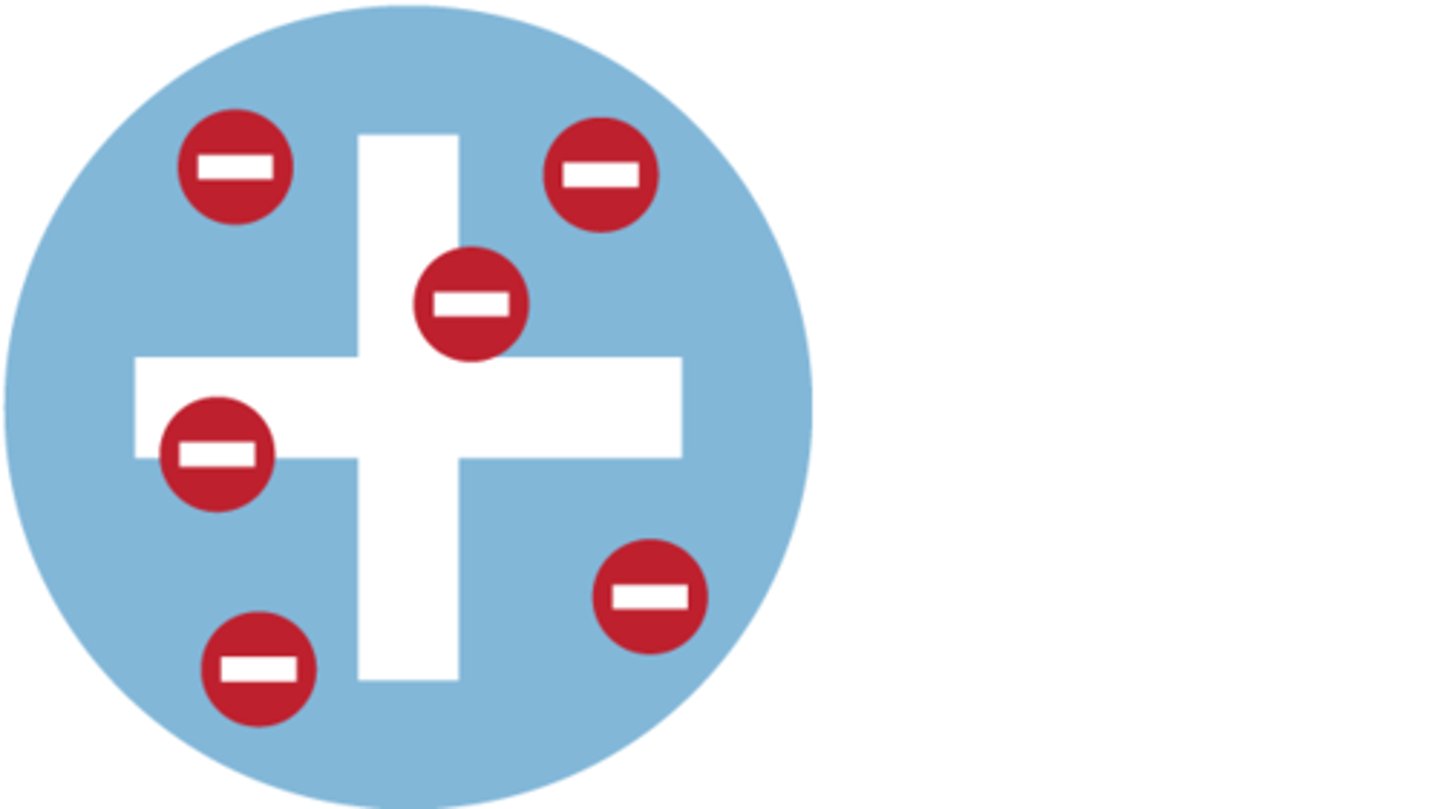
Plum Pudding Model- Positive sphere with electrons dotted around and embedded within- electrons are the plums
JJ Thompson
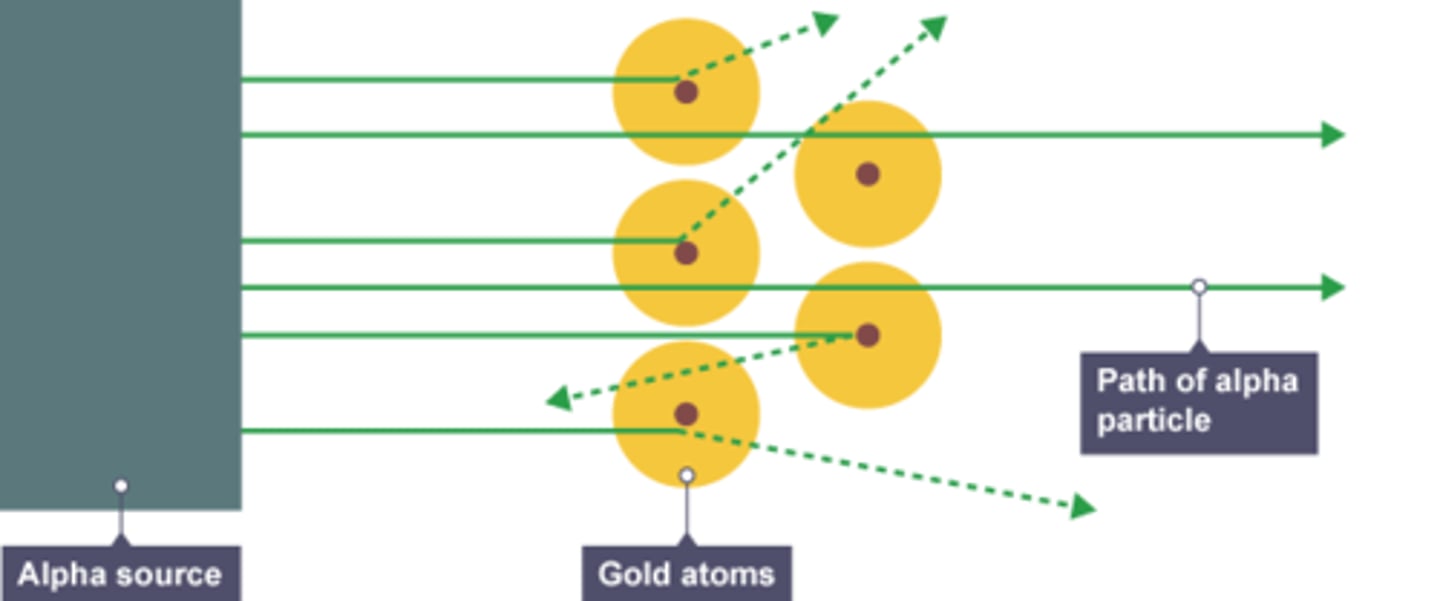
Experiment which caused developments
Alpha Particle Scattering experiment- Alpha particles were fired at gold foil (they have a positive charge). It was expected that the majority of the alpha particles would've been reflected , if the plum pudding model was true
However, the majority passed through, proving atoms were mostly empty space. A few were deflected and a few were reflected proving the positive charge was concentrated in the centre in the nucleus, and that the mass was concentrated in the centre of the atom
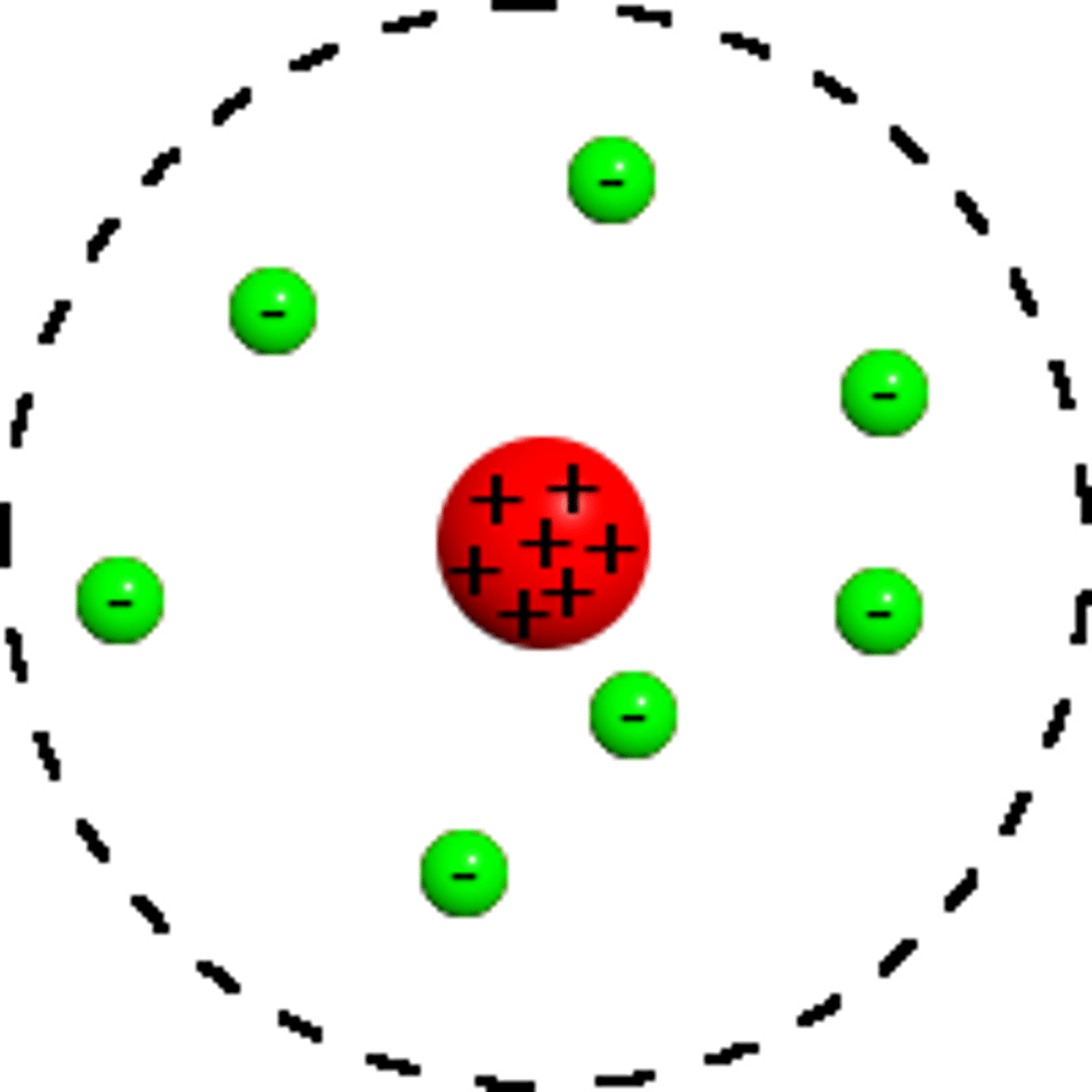
Third model of the atom
Nuclear Model- Positive nucleus surrounded by electrons orbiting
Rutherford
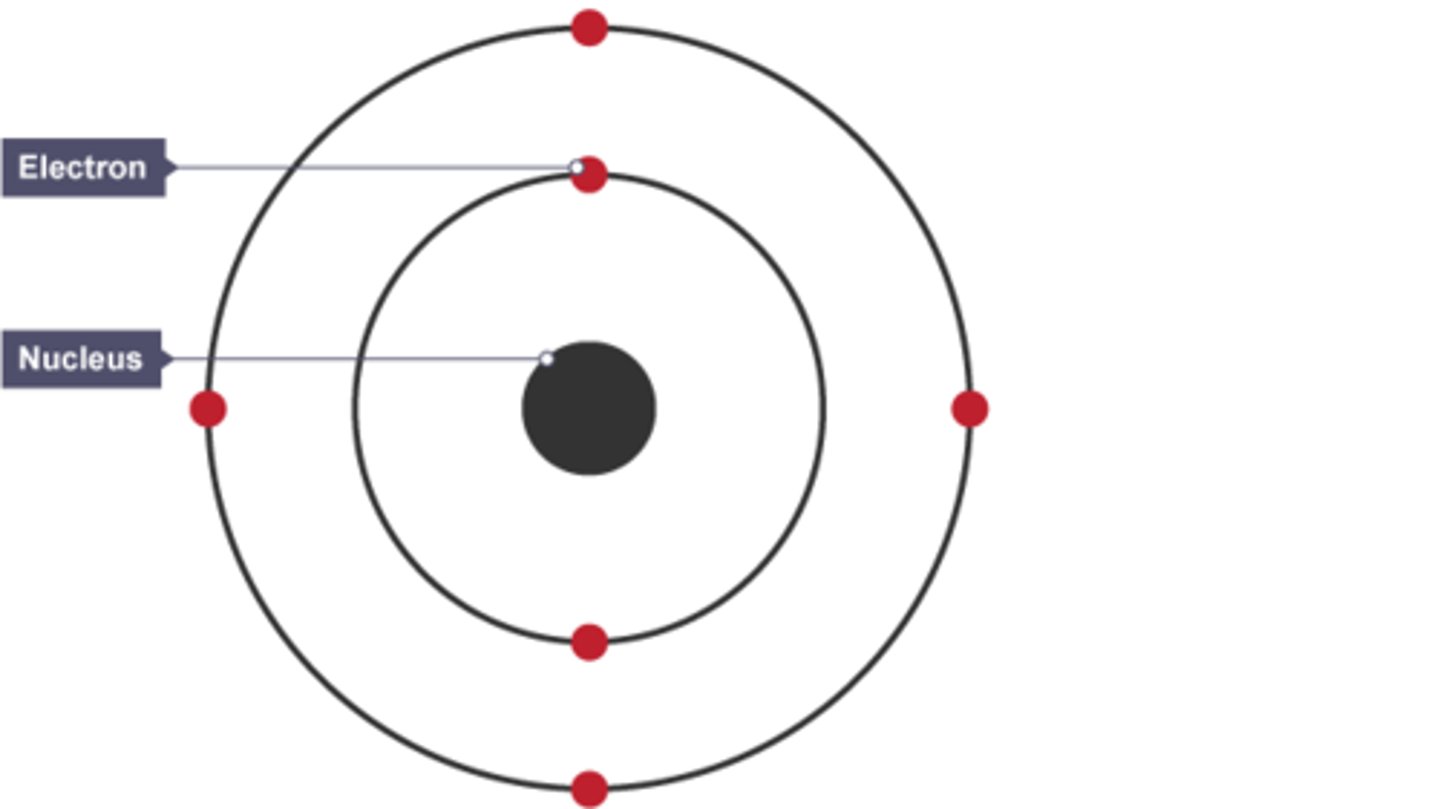
Fourth model of the atom
Niels Bohr adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observations.
What was later discovered about the nucleus
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
Final model of the atom
The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
Radius of an atom in m
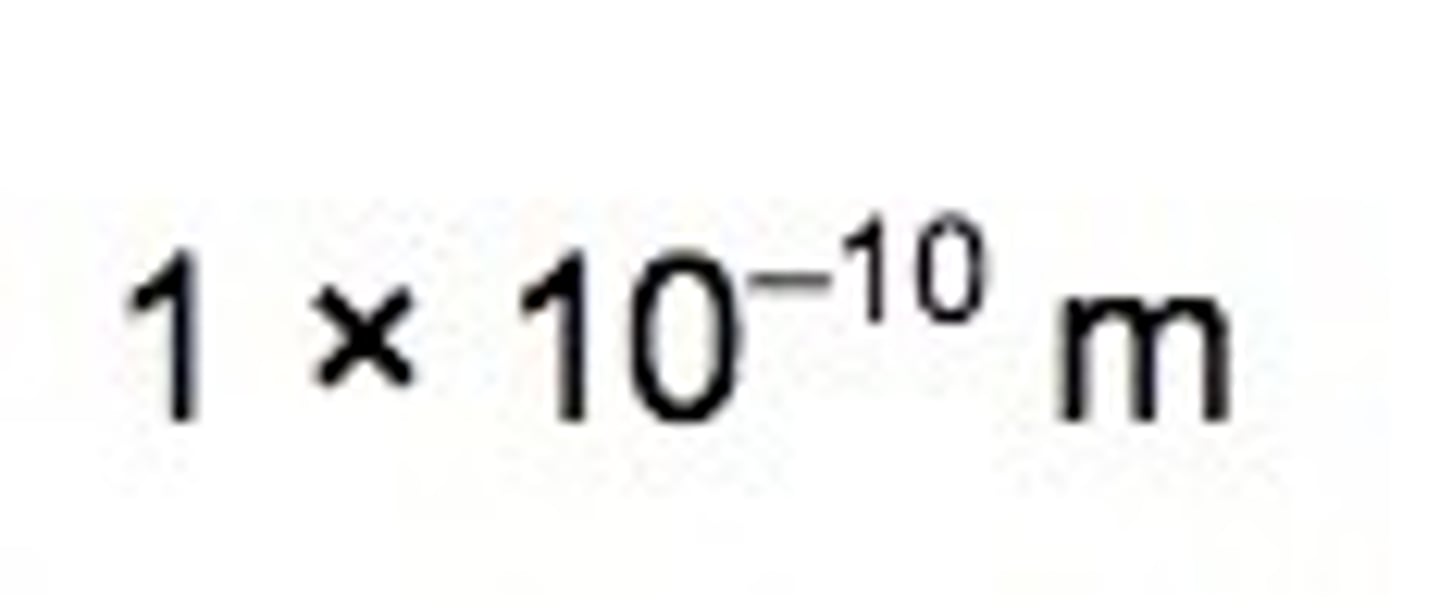
Radius of a nucleus in m
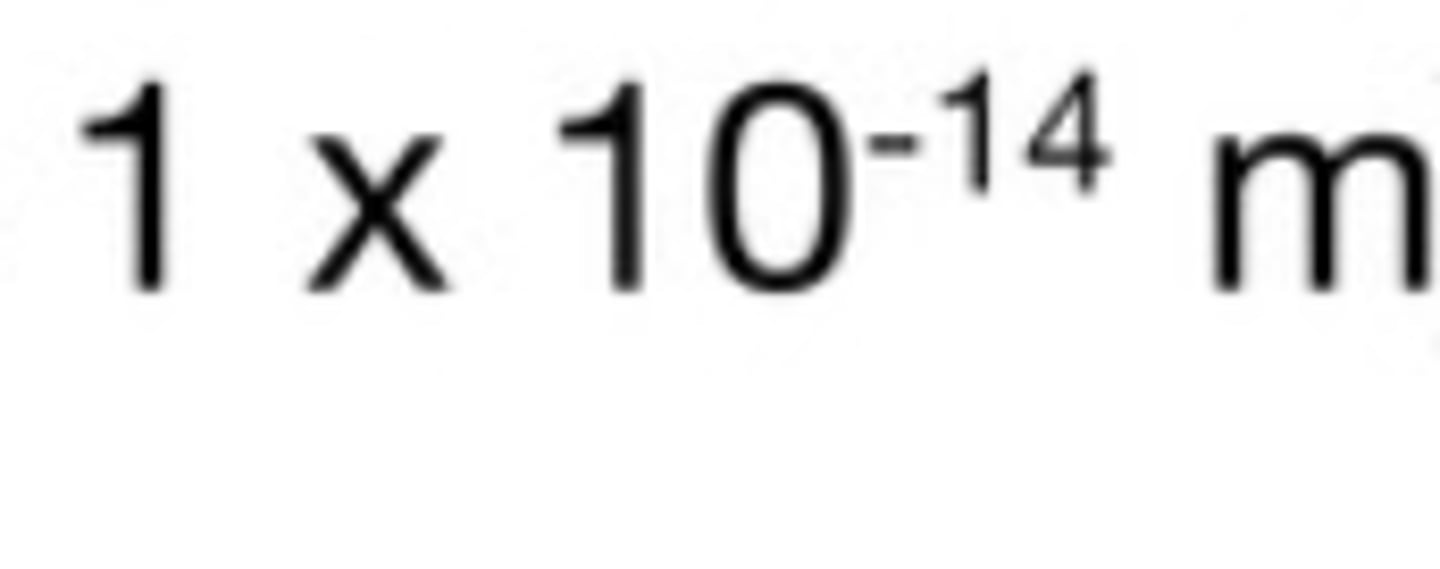
Charge and mass of a proton
Charge = +1
Mass = 1
Charge and mass of a neutron
Charge = 0
Mass = 1
Charge and mass of an electron
Charge= -1
Mass= 0
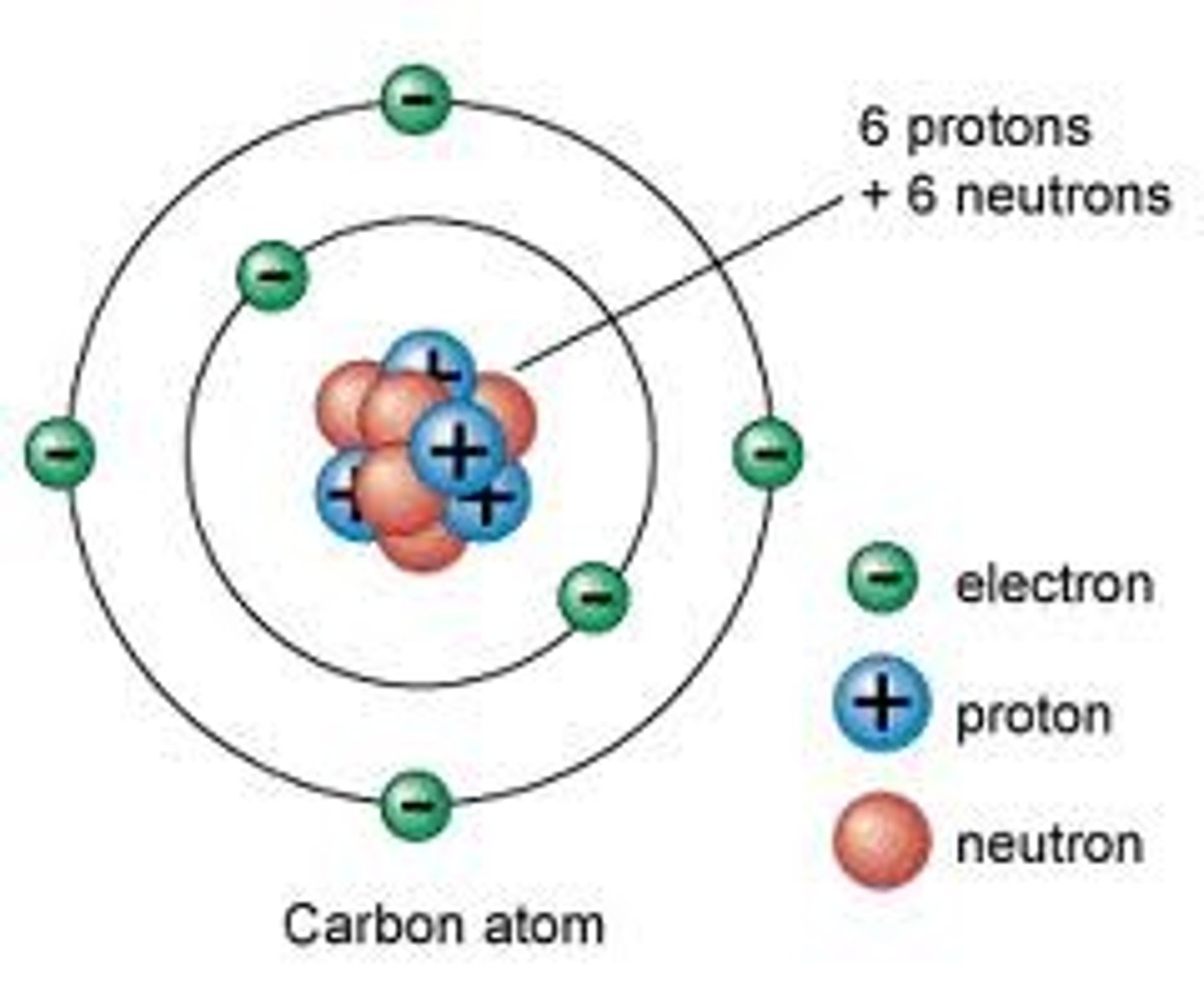
Describe the basic structure of an atom
The basic structure of an atom is a positively charged nucleus composed of both protons and neutrons surrounded by negatively charged electrons.
The radius of a nucleus is less than 1/10 000 of the radius of an atom. Most of the mass of an atom is concentrated in the nucleus.
The electrons are arranged at different distances from the nucleus (different energy levels).
The electron arrangements may change with the absorption of electromagnetic radiation (move further from the nucleus; a higher energy level) orby the emission of electromagnetic radiation (move closer to the nucleus; a lower energy level).
Describe how electrons can move between energy levels
Electrons can absorb electromagnetic radiation, gain a higher energy level, overcome the electrostatic forces of attraction between the nucleus and itself to an extent, and move to a higher energy level
Eventually, the electron is pulled back by the forces of attraction, so releases electromagnetic radiation to reduce its energy levels and move back to a lower energy level
Isotope
Atoms of the same element with different numbers of neutrons
Mass number
The sum of total neutrons and protons in an atom
The mass of an atom relative to 1/12 of a carbon-12 atom
What happens if a nucleus is unstable
Some atomic nuclei are unstable.
The nucleus gives out radiation as it changes to become more stable.
This is a random process called radioactive decay.
What is the activity
Activity is the rate at which a source of unstable nuclei decays .
Activity is measured in becquerel (Bq)
What is count rate
Count-rate is the number of decays recorded each second by a detector (eg Geiger-Muller tube).
What are the 4 types of nuclear radiation
an alpha particle (α) – this consists of two neutrons and two protons, it is the same as a helium nucleus
a beta particle (β) – a high speed electron ejected from the nucleus as a neutron turns into a proton
a gamma ray (γ) – electromagnetic radiation from the nucleus
a neutron (n).
Describe the features of alpha (α) radiation
2 protons and 2 neutrons (a helium nucleus)
Minimum absorption material- sheet of paper
Range in air- 2-3cm
Ionising power- Strong
Penetration - weak
Used in smoke detectors
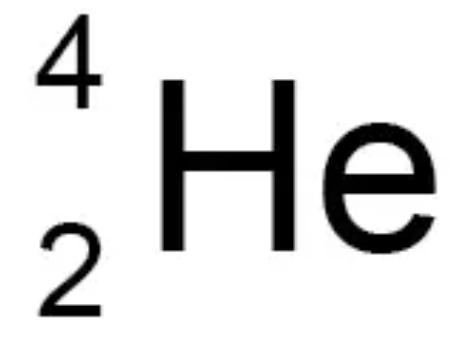
Describe the features of beta (β) radiation
Electron
Created when a neutron emits an electron to become a proton
Minimum absorption material- sheet of aluminium
Range in air- 2-3m
Ionising power- Medium
Penetration - Medium
Used in material thickness testing
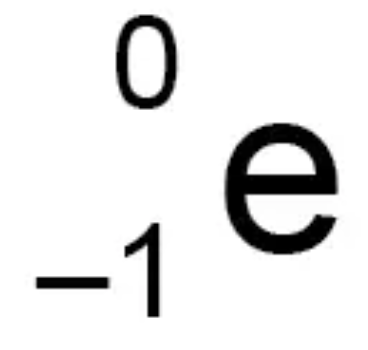
Describe the features of gamma (γ) radiation
Electromagnetic radiation from the nucleus
Minimum absorption material- Thick sheets of lead
Range in air- about 90m
Ionising power- Weak
Penetration power- Strong
Used in medical tracers
Rules for alpha decay equations
Mass number decreases by 4
Atomic number decreases by 2
Rules for beta decay equations
- Mass number remains the same
- Atomic number increases by 1
Nuclear decay equation for Radon (Rn)-219 decaying alpha particles
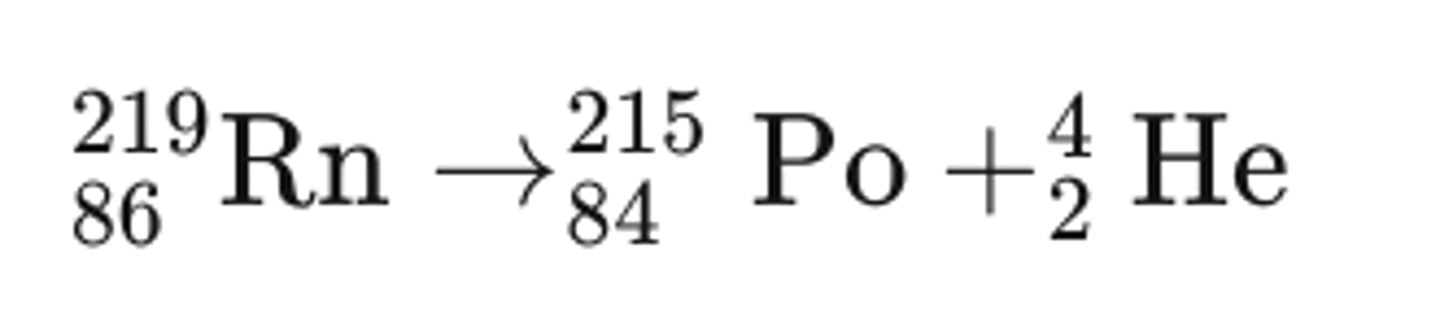
Nuclear decay equation for Carbon (C)-14 decaying beta particles
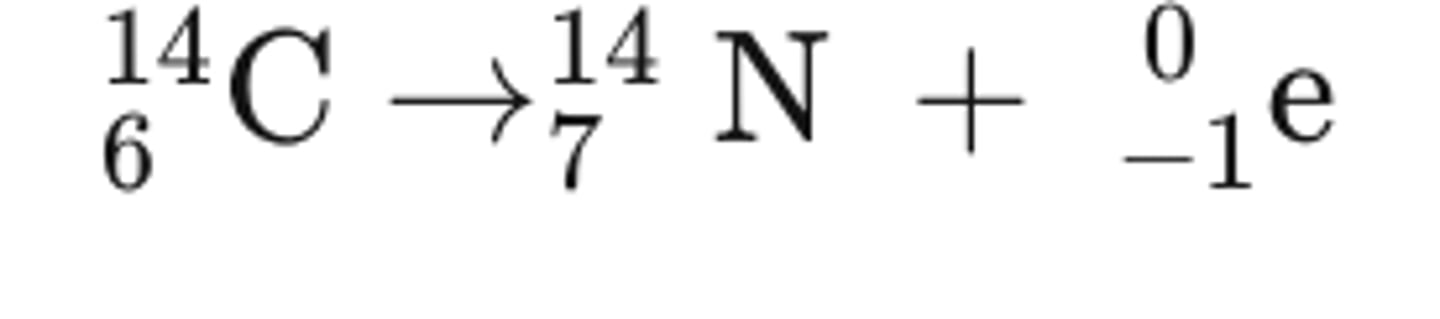
What is Radioactive Contamination
Radioactive contamination is the unwanted presence of materials containing radioactive atoms on other materials.
The hazard from contamination is due to the decay of the contaminating atoms.
The type of radiation emitted affects the level of hazard.
What is Irradiation
Irradiation is the process of exposing an object to nuclear radiation.
The irradiated object does not become radioactive.
What are the precautions against radiation
Keep sources in lead lined boxes
Stand behind barriers or be in a different room to the source
Handle sources with remote controlled arms
Protective equipment such as lead aprons
Contamination showers
What is Half life
The half-life of a radioactive isotope is the time it takes for the number of nuclei of the isotope in a sample to halve, or the time it takes for the count rate (or activity) from a sample containing the isotope to fall to half its initial level.
What is the random nature of radioactive decay
It cannot be predicted when a nucleus will decay
It cannot be predicted which nucleus will decay next
What is the half-life of a sample where the activity drops from 1,200 Bq down to 300 Bq in 10 days?
1200/300=4
4= 2 half lives (2^2=4)
2 half lives=10 days
1 half life = 5 days
Risk of radiation to humans
Radiation can enter cells and ionise atoms within it, causing DNA to mutate, which can cause cancer, or causing the cell to die
Which is most and least dangerous inside the body
Alpha most dangerous
Gamma least dangerous
Which is most and least dangerous outside the body
Gamma most dangerous
Alpha least dangerous
What does a short half life mean
Isotope will decay faster- emits high amounts of radiation to start off with but becomes safer quickly
What does a long half life mean
Isotope will decay slower- emits low amounts of radiation over a longer period of time, nearby areas are exposed for longer
What is background radiation and where does it come from
Background radiation is around us all of the time. It comes from:
natural sources such as rocks and cosmic rays from space
man-made sources such as the fallout from nuclear weapons testing, medical imaging (eg x rays) and nuclear accidents like Chernobyl.
The level of background radiation and radiation dose may be affected by occupation and/or location.
What is radiation dose, and what is it measured in
The risk of harm to the body tissues due to exposure to radiation
Measured in Sieverts (Sv)
What does dose depend on
- Job
- Living location
What is the maximum dosage per year for workers
20mSv
What can employers do to prevent too much dosage for their workers
Schedule workers shifts so that no worker receives too much at a time
Use badges that change colour based on how much radiation has been absorbed
What type of radiation should be used for detecting cancers, and what should the half life be
Gamma radiation- Easier to detect outside the body, and the least dangerous inside the body
Short Half life- The inside of the body is exposed to radiation for a shorter amount of time- less harm caused
What type of radiation should be used for a smoke detector
Alpha- Smoke will stop the alpha particles, so the geiger counter will notice the difference when smoke is present
Long half life- It won't have to be replaced very often
What is nuclear radiation used for in medicine
Nuclear radiations are used in medicine for the:
exploration of internal organs
control or destruction of unwanted tissue.
What is nuclear fission
Nuclear fission is the splitting of a large and unstable nucleus (eg uranium or plutonium).
What are the steps involved in fission
Spontaneous fission is rare.
Usually , for fission to occur the unstable nucleus must first absorb a neutron.
The nucleus undergoing fission splits into two smaller nuclei, roughly equal in size, and emits two or three neutrons plus gamma rays.
Energy is released by the fission reaction.
All of the fission products have kinetic energy.
The neutrons may go on to start a chain reaction.
Why must the chain reaction be controlled in fission
The neutrons may go on to start a chain reaction.
The chain reaction is controlled in a nuclear reactor to control the energy released.
The explosion caused by a nuclear weapon is caused by an uncontrolled chain reaction.
What is nuclear fusion
Nuclear fusion is the joining of two light nuclei to form a heavier nucleus.
In this process some of the mass may be converted into the energy of radiation.