Medical physics - x-rays
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
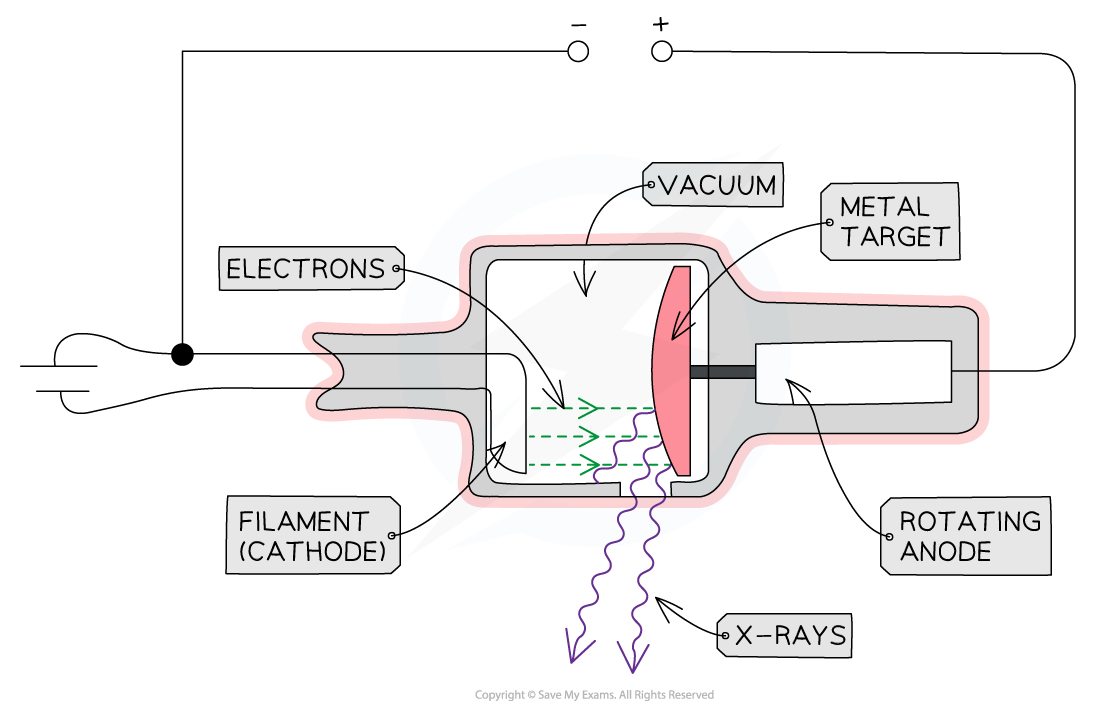
Structure of an X-ray tube:
A heated cathode
An anode
A metal target
A high voltage power supply
Use:
Medical imaging (radiography)
Security
Industrial imaging
What does heated cathode do:
At one end of the tube is the cathode (negative terminal) which is heated by an electric current
The heat causes electrons to be liberated from the cathode, gathering in a cloud near its surface
This process of thermionic emission is the source of the electrons
Anode use:
At the other end of the tube, an anode (positive terminal) is connected to the high voltage supply
This allows the electrons to be accelerated up to a voltage of 200 kV
When the electron arrives at the anode, its kinetic energy is 200 keV (by the definition of an electronvolt)
Only about 1% of the kinetic energy is converted to X-rays
The rest is converted to heat energy
Therefore, to avoid overheating, the anode is spun at 3000 rpm and sometimes water-cooled
tube surrounded by lead shielding:
This is to ensure the safety of the operators and recipients of the X-rays
An adjustable window allows a concentrated beam of X-rays to escape and be controlled safely
housed in vacuum chamber:
This is to ensure that the electrons do not collide with any particles on their way to the metal target
When the fast-moving electrons collide with the target, X-rays are produced by one of two methods:
Method 1: Bremsstrahlung
Method 2: Characteristic Radiation
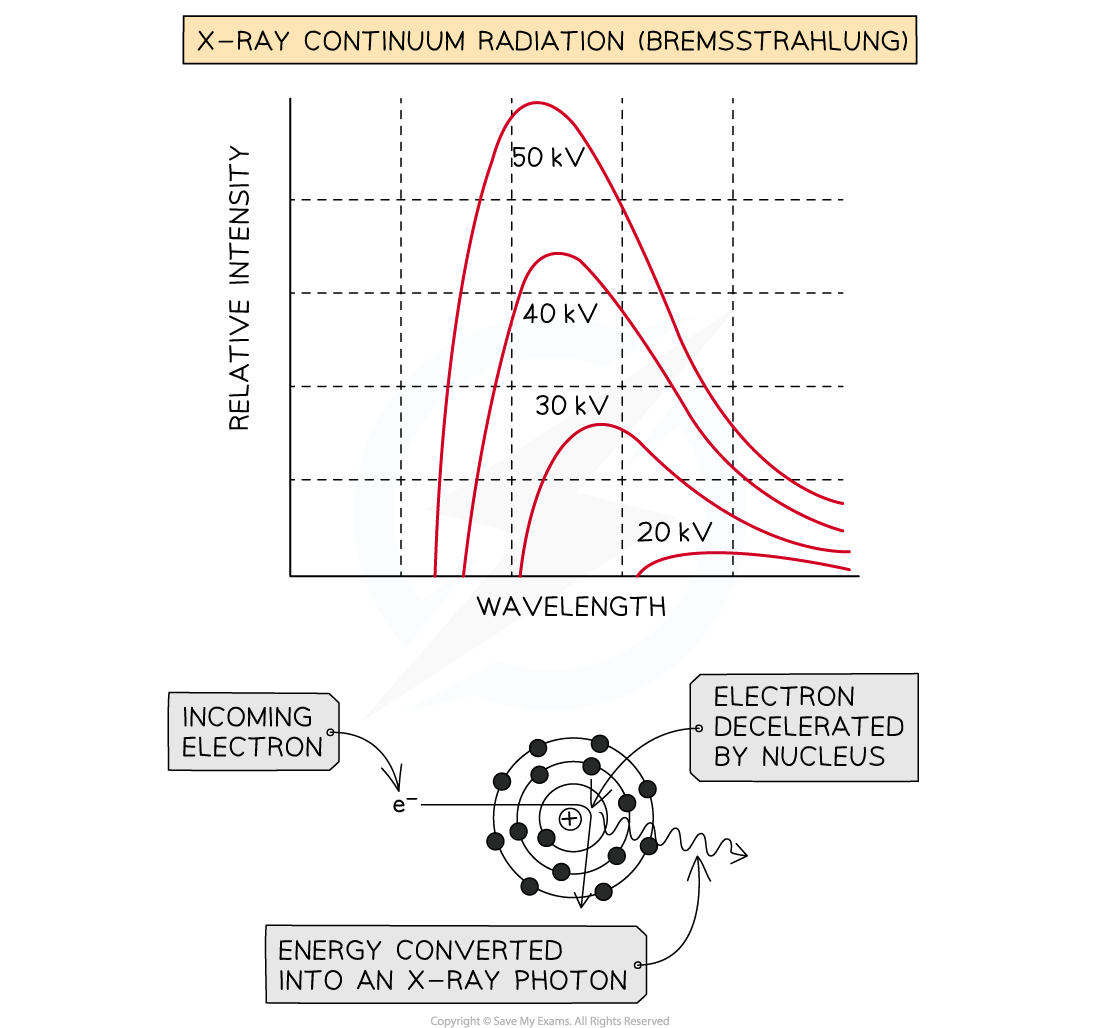
Method 1: Bremsstrahlung
When the high-speed electrons collide with the metal target, they undergo a steep deceleration
When a charged particle decelerates quickly, some of the energy released is converted into a photon
A small amount of the kinetic energy (~ 1%) from the incoming electrons is converted into X-rays as the electrons decelerate in the tungsten, due to conservation of energy
The rest of the energy heats up the anode, which usually requires some form of cooling
The energy of the X-ray photon can be of any value, up to the original kinetic energy of the electron, giving a spread of possible X-ray energies
These X-rays cause the continuous or ‘smooth hump shaped’ line on an intensity wavelength graph
what is the maximum energy of electron equal to when accelerated:
it gains energy equal to the electronvolt, this energy can be calculated using:
Emax = eV
What is its wavelength
The smallest possible wavelength is equivalent to the highest possible frequency and therefore, the highest possible energy
This is assuming all of the electron’s kinetic energy has turned into electromagnetic energy
Therefore, the maximum X-ray frequency fmax, or the minimum wavelength λmin, that can be produced is calculated using the equation:
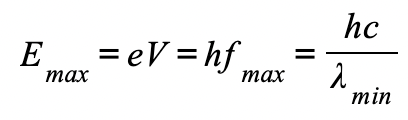
The maximum X-ray frequency, fmax, is therefore equal to:
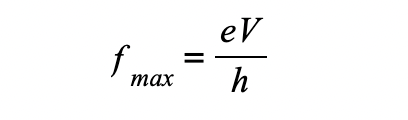
The minimum X-ray wavelength, λmin, is therefore equal to:
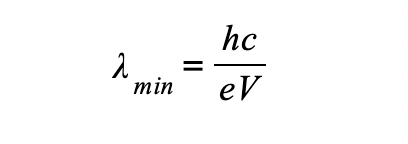
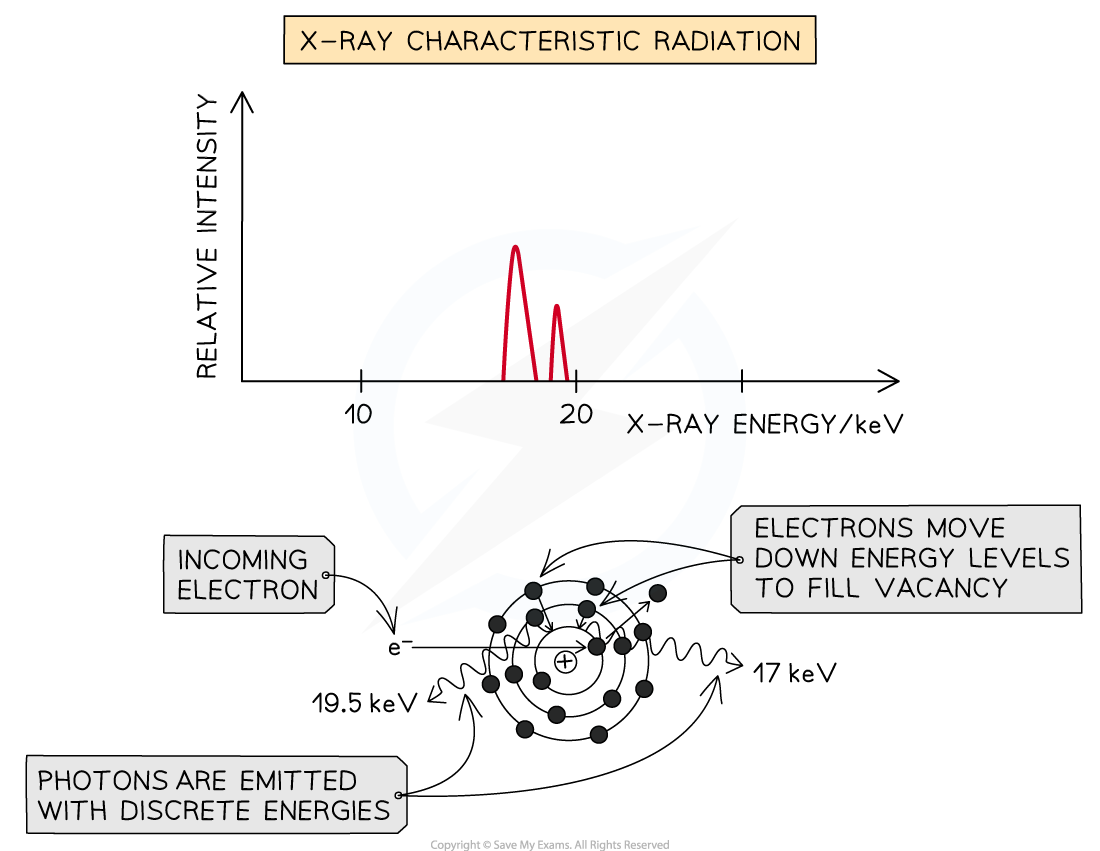
Method 2: Characteristic Radiation
Some of the incoming fast electrons cause inner shell electrons of the tungsten to be ‘knocked out’ of the atom, leaving a vacancy
This vacancy is filled by an outer electron moving down and releasing an X-ray photon as it does (equal in energy to the difference between the two energy levels)
Because these X-rays are caused by energy level transitions, they have only specific discrete energies
They cause sharp spikes on an intensity wavelength graph
The number of spikes depends on the element used for the target - there are two sets of spikes for a tungsten target, representing two sets of possible energy transitions
Explain why:
a) A continuous spectrum of wavelengths is produced.
Step 1: Consider the path of the electrons from the cathode to the anode
Photons are produced whenever a charged particle undergoes a large acceleration or deceleration
X-ray tubes fire high-speed electrons at a metal target
When an electron collides with the metal target, it loses energy in the form of an X-ray photon as it decelerates
Step 2: Consider the relationship between the energy of the electron and the wavelength of the photon
The wavelength of a photon depends on the energy transferred by a decelerating electron
The electrons don't all undergo the same deceleration when they strike the target
This leads to a distribution of energies, hence, a range, or continuous spectrum, of wavelengths is observed
explain why:
b) The gradient is steeper at shorter wavelengths.
Step 1: Identify the significance of the intensity
The intensity of the graph signifies the proportion of photons produced with a specific energy, or wavelength
The higher the intensity, the more photons of a particular wavelength are produced
In other words, the total intensity is the sum of all the photons with a particular wavelength
Step 2: Explain the shape of the graph
When a single electron collides with the metal target, a single photon is produced
Most electrons only give up part of their energy, and hence there are more X-rays produced at wavelengths higher than the minimum (or energies lower than the maximum)
At short wavelengths, there is a steeper gradient because only a few electrons transfer all, or most of, their energy
explain why:
c) The spectrum has a sharp cut-off at short wavelengths.
Step 1: Identify the relationship between minimum wavelength and maximum energy
The minimum wavelength of an X-ray is equal to
λmin=hc/Emax
The equation shows the maximum energy of the electron corresponds to the minimum wavelength, they are inversely proportional
λmin∝1/Emax
Therefore, the higher the energy of the electron, the shorter the wavelength of the X-ray produced
Step 2: Explain the presence of the cut-off point
The accelerating voltage determines the kinetic energy which the electrons have before striking the target
The value of this accelerating voltage, therefore, determines the value of the maximum energy
This corresponds to the minimum, or cut-off, wavelength
X-ray attenuation is defined as:
The reduction in energy, or intensity, of a beam of X-rays due to their interaction with matter (occurs within material the X-ray is travelling in)
There are four main methods in which X-rays can be attenuated:
Simple scattering
Photoelectric effect
Compton scattering
Pair production
Simple scattering occurs when:
A low-energy X-ray photon encounters an electron in an atom causing it to be scattered without a change in energy
What does “low energy” mean in this scenario?
energy of X-ray photon is not sufficient to cause ionisation
During simple scattering, photons are deflected from their initial path by interaction with the atoms of the material. However, there are:
No change in energy of the X-ray photon
No absorption of the X-ray photon
What does this cause?
blurring or 'noise' in X-ray imaging
This is because scattered X-rays arrive at the detector from several angles as well as from the main beam
The photoelectric effect occurs when:
An X-ray photon is absorbed by an inner shell electron causing it to be ejected from the atom as a photoelectron
When the X-ray photon is completely absorbed what happens to its energy?
its energy is imparted to the photoelectron
energy is always conserved, the energy of an incident X-ray photon is equal to:
The work function + the maximum kinetic energy of the photoelectron


The Compton Effect is when:
An X-ray photon is deflected by an interaction with an orbital electron causing the wavelength of the photon to increase and the ejection of the electron from the atom at a high speed
How is this process different to simple scattering?
X-ray photon imparts some of its energy to the orbital electron
Why does it impart some energy to orbital electron?
Because of this exchange of energy:
The X-ray is deflected from its initial path
The X-ray’s wavelength increases, as its energy decreases
The electron involved is ejected from the atom involved in the interaction
What direction do the the electron and X-ray move in after + why?
deflect in diff directions
conservation of momentum

Pair production occurs when:
A high energy X-ray photon passes close to the nucleus of an atom causing the production of an electron-positron pair
Why does this occur?
as a consequence of Einstein's mass-energy equivalence principle:
E = mc2
E= energy of X-ray photon
m= mass of electron and position= 2me (kg)
c= speed of light
therefore pair production can only occur with _____ + why?
high energy x-rays
because the energy of the X-ray photon must be above a certain value to provide the total rest mass energy of the electron-positron pair
minimum energy, Emin, for a photon to undergo pair production is the total rest mass energy of the particles produced:
Emin = hfmin = 2mec2
As a result of pair production, the X-ray photon is completely absorbed and all its energy is imparted to the electron-positron pair

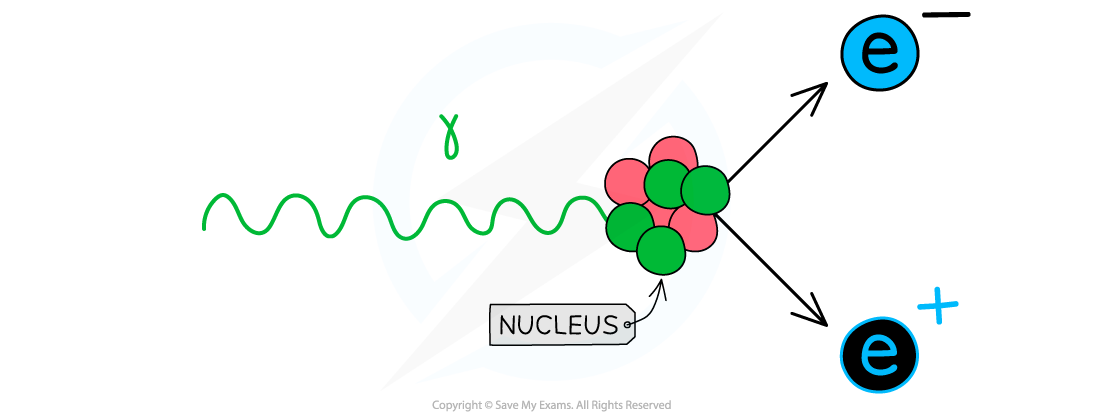
Bones _______ X-ray radiation- this is why they appear white on the X-ray photograph
absorb
What is a collimated beam?
beam of light in which beams of light travel parallel to each other
When the collimated beam of X-rays passes through the patient’s body, they are ________
absorbed and scattered
The attenuation of X-rays can be calculated using the equation:
I = I0 e−μx
I0 = the intensity of the incident beam (W m-2)
I = the intensity of the transmitted beam (W m-2)
μ = the linear absorption coefficient (m-1)
x = distance travelled through the material (m)
The attenuation coefficient also depends on the energy of the X-ray photons
The intensity of the X-ray decays exponentially
The thickness of the material that will reduce the X-ray beam or a particular frequency to half its original value is known as the half thickness
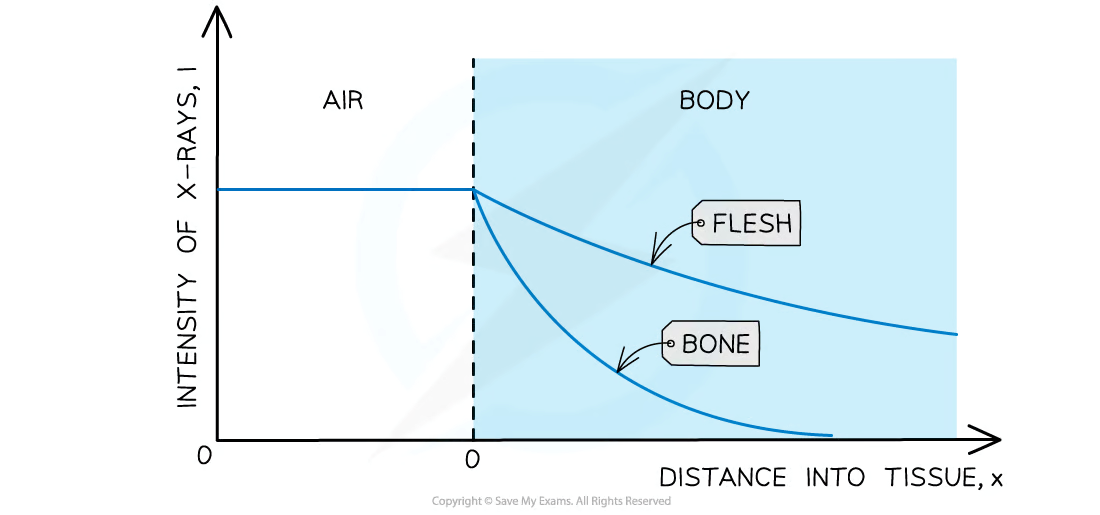
When treating patients, the aims are to:
Reduce the exposure to radiation as much as possible
Improve the contrast of the image
Why can X-rays be bad?
are ionising, meaning they can cause damage to living tissue and can potentially lead to cancerous mutations
What are used to ensure patients experience the least possible exposure?
aluminium filters
Why are aluminium filters used?
This is because many wavelengths of X-ray are emitted
Longer wavelengths of X-ray are less penetrating, therefore, they are more likely to be absorbed by the body
This means they do not contribute to the image and pose more of a health hazard
The aluminium sheet absorbs these long wavelength X-rays making them safer
Contrast is defined as:
The difference in degree of blackening between structures
How can image contrast can be improved?
Using the correct level of X-ray hardness: hard X-rays for bones, soft X-rays for tissue
Using a contrast media
Sharpness is defined as:
How well defined the edges of structures are
How can image sharpness can be improved?
Using a narrower X-ray beam
Reducing X-ray scattering by using a collimator or lead grid
Smaller pixel size
Contrast media is defined as:
A substance, such as barium or iodine, which is a good absorber of X-rays. A patient is given this so it can give a better contrast on an X-ray image
These are sometimes used because:
Some soft tissue organs do not show up on X-rays when the organ has a similar attenuation coefficient to other tissues in the same area
Contrast media are good absorbers of X-rays as they have a large attenuation coefficient
Hence when contrast media enter an organ, the image of the organ is enhanced when imaged using X-rays
When is barium used vs iodine?
Iodine is used as a contrast medium in liquids i.e. to observe blood flow - this is usually injected into the patient
Barium sulphate is used as a contrast medium in the digestive system - this is usually ingested by mouth and is known as a barium meal
What is the large attenuation coefficient of contrast materials due to?
large atomic number of these elements
When is a CAT (CT)/ computerised axial tomography scan needed?
more comprehensive image is needed as 2D x-ray not providing enough information about internal structures
What are the main features of a CT scan?
An X-ray tube rotates around the stationary patient
A CT scanner takes X-ray images of the same slice, at many different angles
This process is repeated, then images of successive slices are combined together
A computer pieces the images together to build a 3D image
This 3D image can be rotated and viewed from different angles

Advantages of CAT scans:
Produces much more detailed images (software can add colour and sharpen images, and parts of the image can be edited out)
Can distinguish between tissues with similar attenuation coefficients giving a higher resolution image
Soft tissue and bone can be imaged in a single process
Produces a 3D image of the body by combining the images at each direction
No overlapping images (for example bones obscuring organs)
Disadvantages of CAT scans:
The patient receives a much higher dose than a normal X-ray
Possible side effects from the contrast media
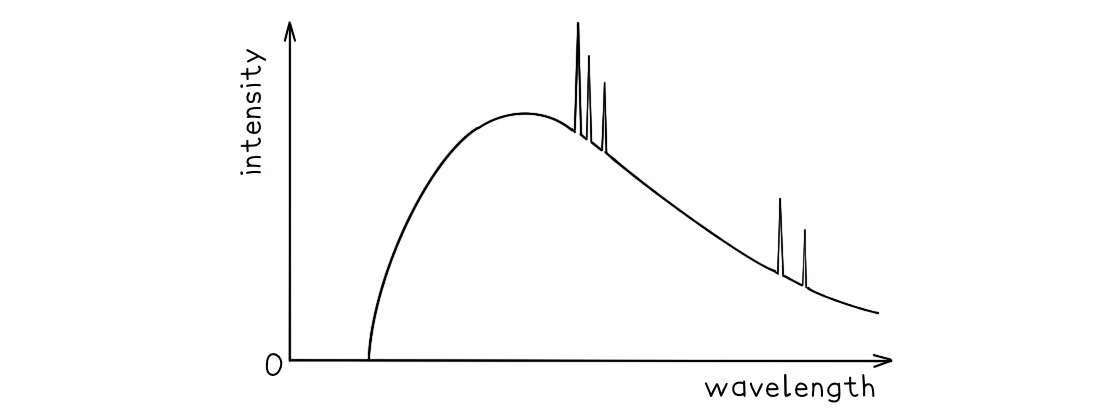
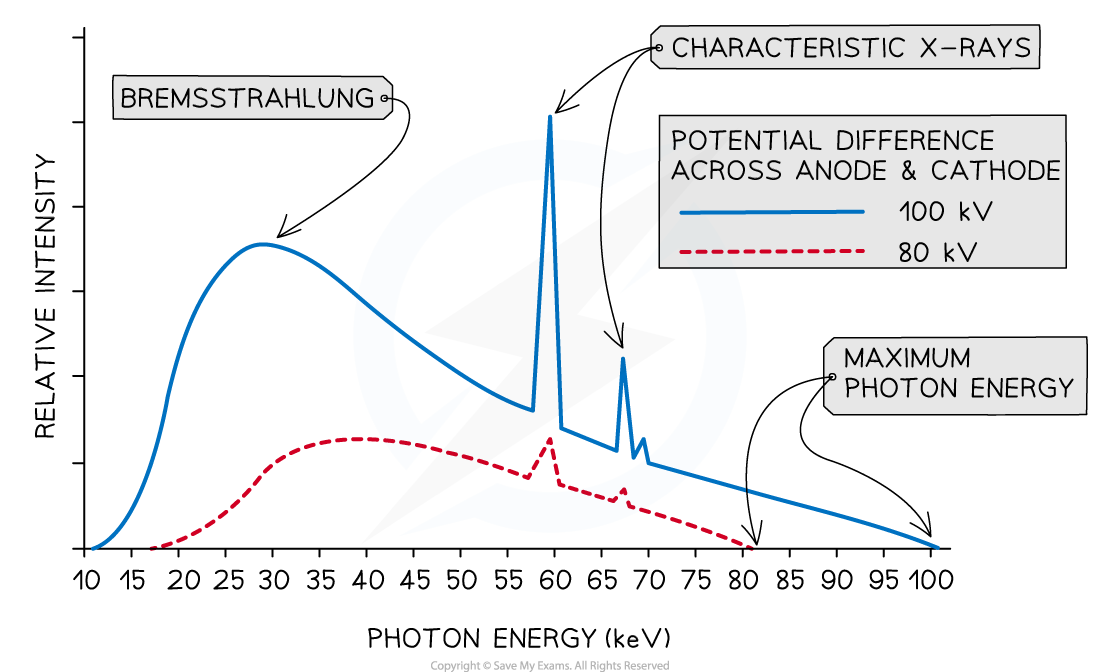
A typical spectrum of the X-ray radiation produced by electron bombardment of a metal target is shown below.
Explain why:
a) A continuous spectrum of wavelengths is produced.
Step 1: Consider the path of the electrons from the cathode to the anode
Photons are produced whenever a charged particle undergoes a large acceleration or deceleration
X-ray tubes fire high-speed electrons at a metal target
When an electron collides with the metal target, it loses energy in the form of an X-ray photon as it decelerates
Step 2: Consider the relationship between the energy of the electron and the wavelength of the photon
The wavelength of a photon depends on the energy transferred by a decelerating electron
The electrons don't all undergo the same deceleration when they strike the target
This leads to a distribution of energies, hence, a range, or continuous spectrum, of wavelengths is observed
The gradient is steeper at shorter wavelengths.
Step 1: Identify the significance of the intensity
The intensity of the graph signifies the proportion of photons produced with a specific energy, or wavelength
The higher the intensity, the more photons of a particular wavelength are produced
In other words, the total intensity is the sum of all the photons with a particular wavelength
Step 2: Explain the shape of the graph
When a single electron collides with the metal target, a single photon is produced
Most electrons only give up part of their energy, and hence there are more X-rays produced at wavelengths higher than the minimum (or energies lower than the maximum)
At short wavelengths, there is a steeper gradient because only a few electrons transfer all, or most of, their energy
The spectrum has a sharp cut-off at short wavelengths.
Step 1: Identify the relationship between minimum wavelength and maximum energy
The minimum wavelength of an X-ray is equal to
λmin=hc/Emax
The equation shows the maximum energy of the electron corresponds to the minimum wavelength, they are inversely proportional
λmin∝1/Emax
Therefore, the higher the energy of the electron, the shorter the wavelength of the X-ray produced
Step 2: Explain the presence of the cut-off point
The accelerating voltage determines the kinetic energy which the electrons have before striking the target
The value of this accelerating voltage, therefore, determines the value of the maximum energy
This corresponds to the minimum, or cut-off, wavelength