Chemistry Buzz Words
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Coulombic Attraction
Discussing the attraction of atoms due to differences in charge, when unlike charges attract each other or when like charges repel each other
Electronegativity
Tendency of an atom to draw the shared electrons in a molecule towards itself
Bond Polarity
Separation of an electric charge along a bond based on the individual atoms electronegativity —> leads to electric dipoles aka asymmetric changes, can be polar or non polar
Polar Covalent Bonds
Between 2 nonmetals that have different enough electronegativities so the bonding electron pair is not equally shared
Non-polar Covalent Bonds
Electronegativities of each atom are so similar that sharing of electron density is equal throughout the molecule
Intramolecular forces
The relation between two atoms in a bond
Intermolecular forces
The relation between two compounds
Spectroscopy
Studies of electromagnetic spectrum with matter
Elemental Analysis
Process where a sample of some material is analyzed for it’s elemental and sometimes isotopic composition
Photoelectric effect
emission of electrons when electromagnetic radiation hits a material
Ionization energy
Minimum amount of energy used to to remove the most loosely bounded electron, valence, of an isolated neutral gaseous atom or molecule (KJ/mol or kcal/mol)
Binding energy
The minimum amount of energy to remove all the electrons from the atom or ion in it’s ground state
Effective Nuclear Charge
Net positive charge pulling electrons towards the nucleus, the positive charge of nuclear protons acting on valence electrons
Atomic Radius
Distance between the center of the nucleus and the boundary of the surrounding shells of the electrons
Electron Affinity
Amount of energy released when an electron is added to a neutral atom or molecule in gaseous state to form negative ion
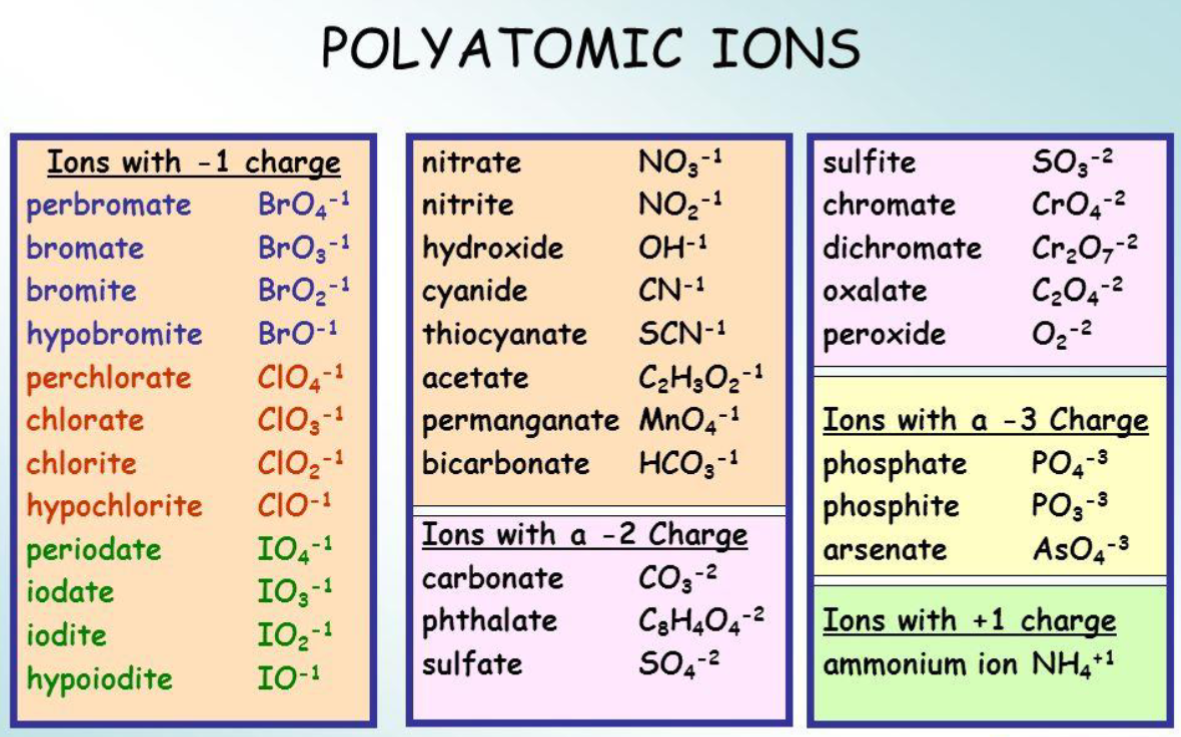
Polyatomic ions
Just know them
Relative Intensity
Amount of an ion produced in relation to the amount of most abundant ion, represents chemical element abundance
Fragmentation
Breaking a molecule apart by ripping the electrons off
Free Radical
The left out electron in odd electron species
VSEPR Theory (valence shell electron pair repulsion)
Electron groups repel each other through coulombic forces
Dipole moment
Difference in electron density, one has more pull than another, determines polarity of a molecule
Coulombs Law
Used to determine the force between point charges
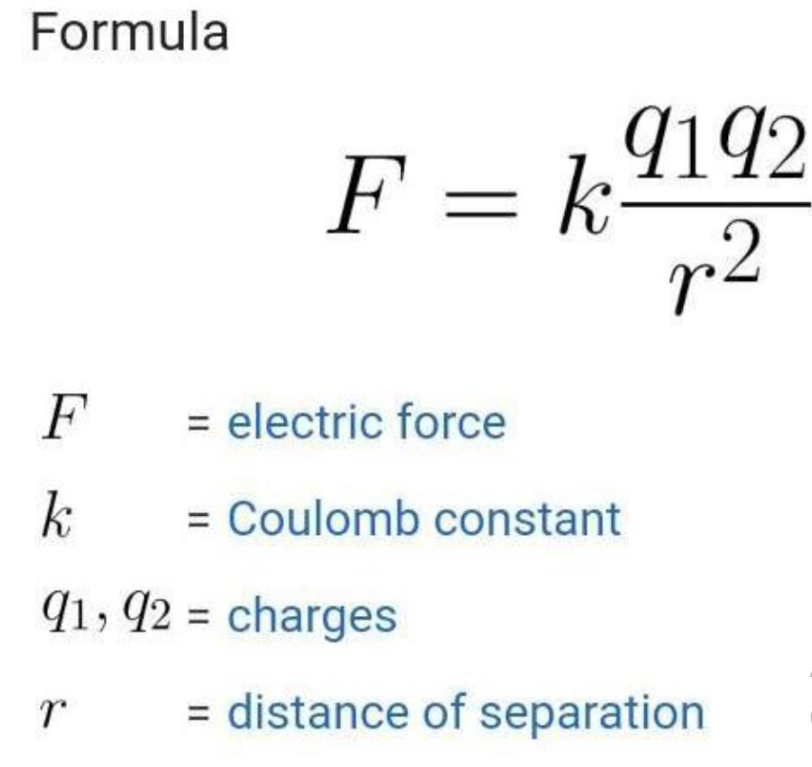
Electromagnetic Spectru
All of the different possible wavelengths of light (electromagentic radiation)