Savvas Biology 2.3 and 2.4 - The Chemistry of Life
1/144
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
145 Terms
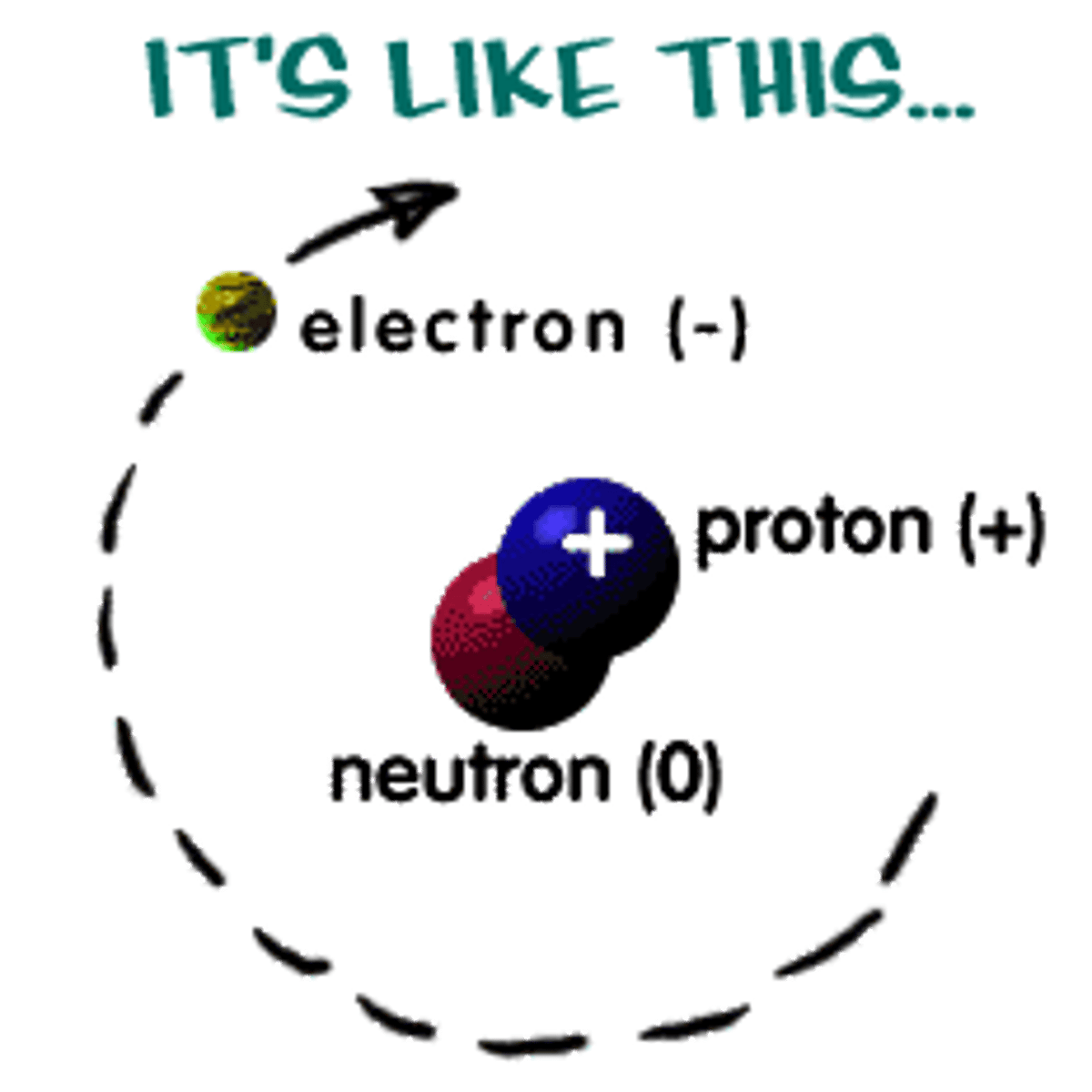
atom
the building blocks of matter; contains subatomic particles: neutrons, electrons, and protons
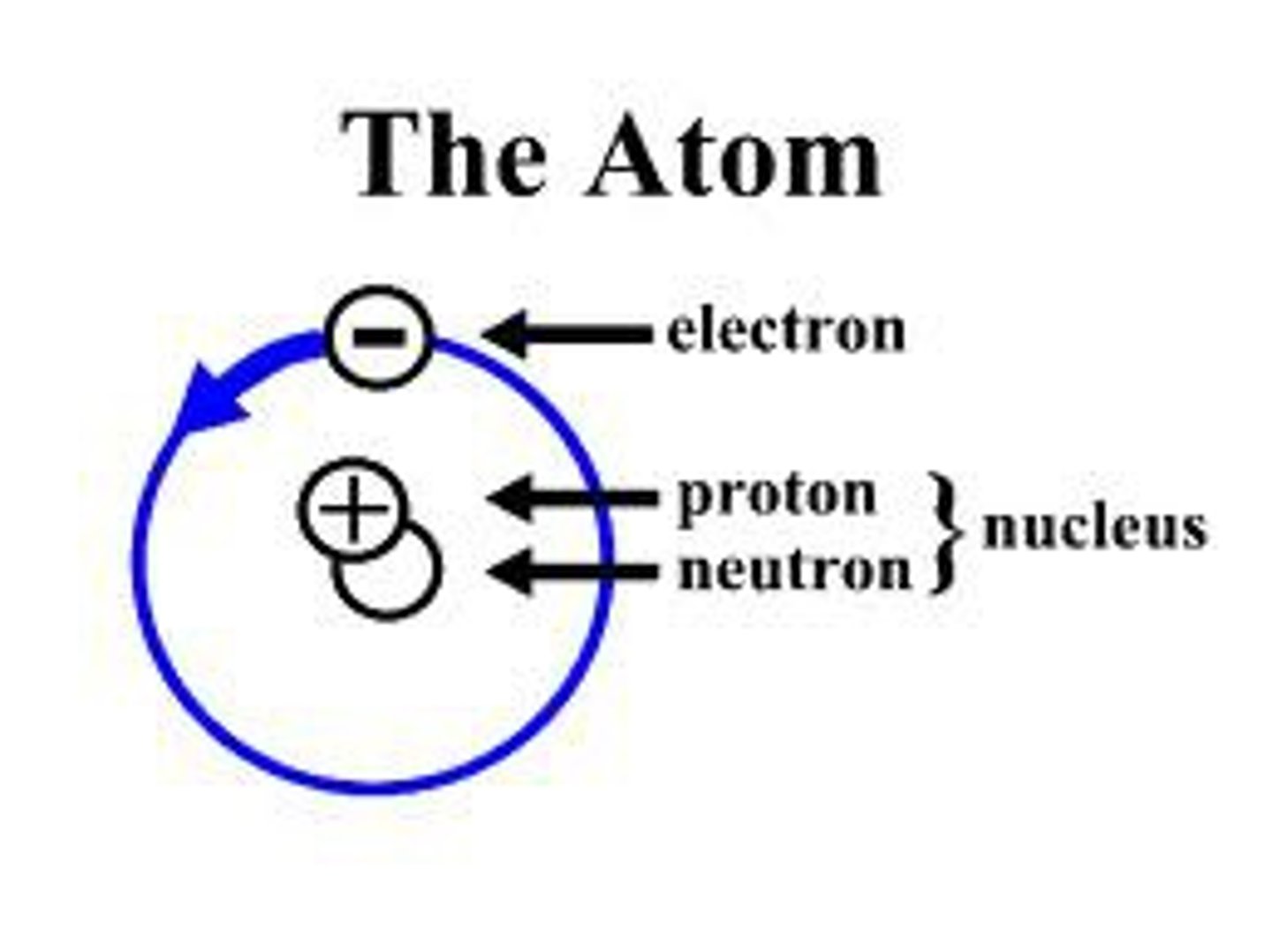
nucleus
located at the center of the atom, consists of protons and neutrons
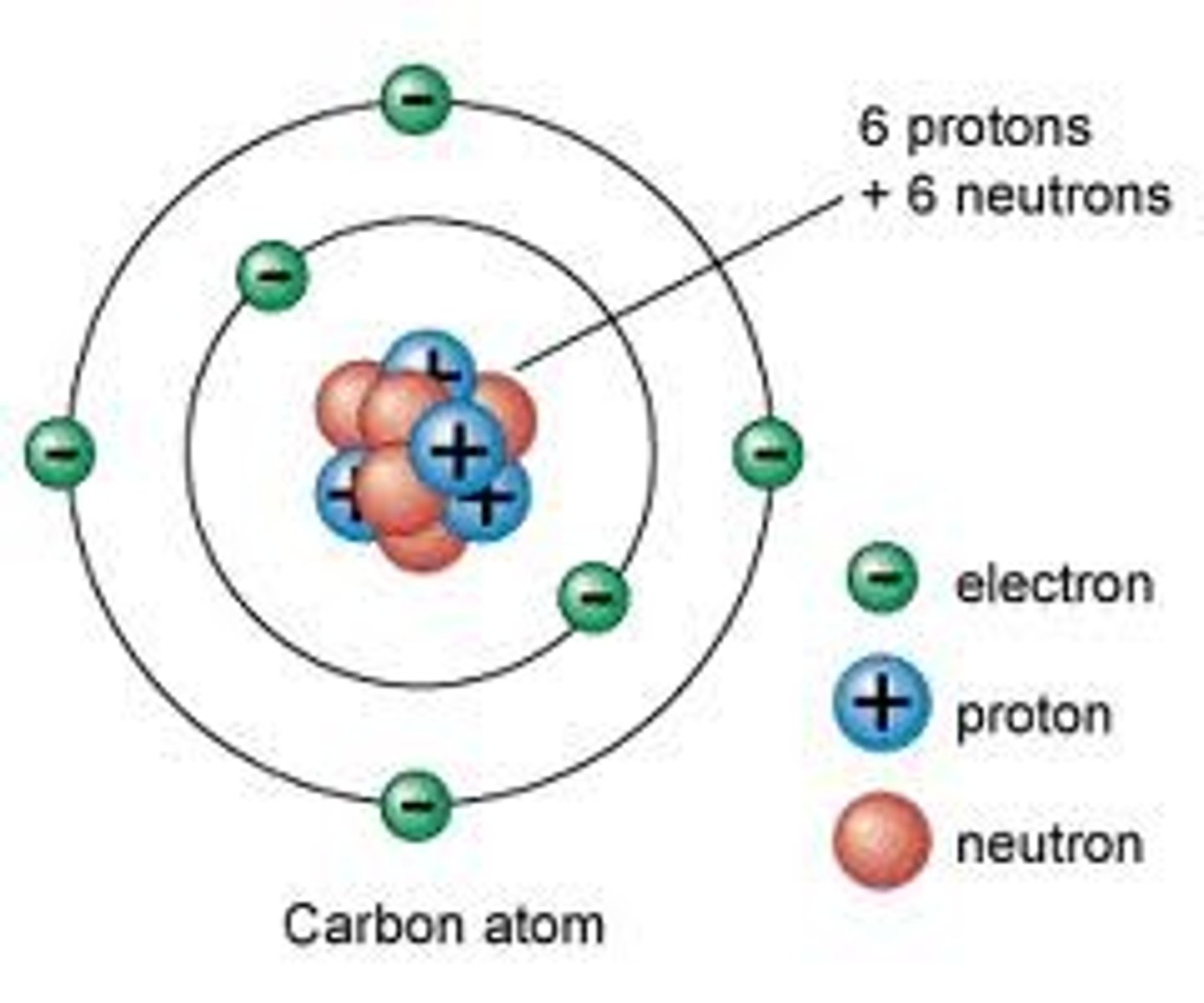
proton
positively charged particle located in the center of an atom
electron
negatively charged particle located outside the nucleus
neutron
particle with no charge located in the center of an atom
element
A pure substance composed of only one type of atom. A pure substance that cannot be broken down into other substances by physical or chemical means
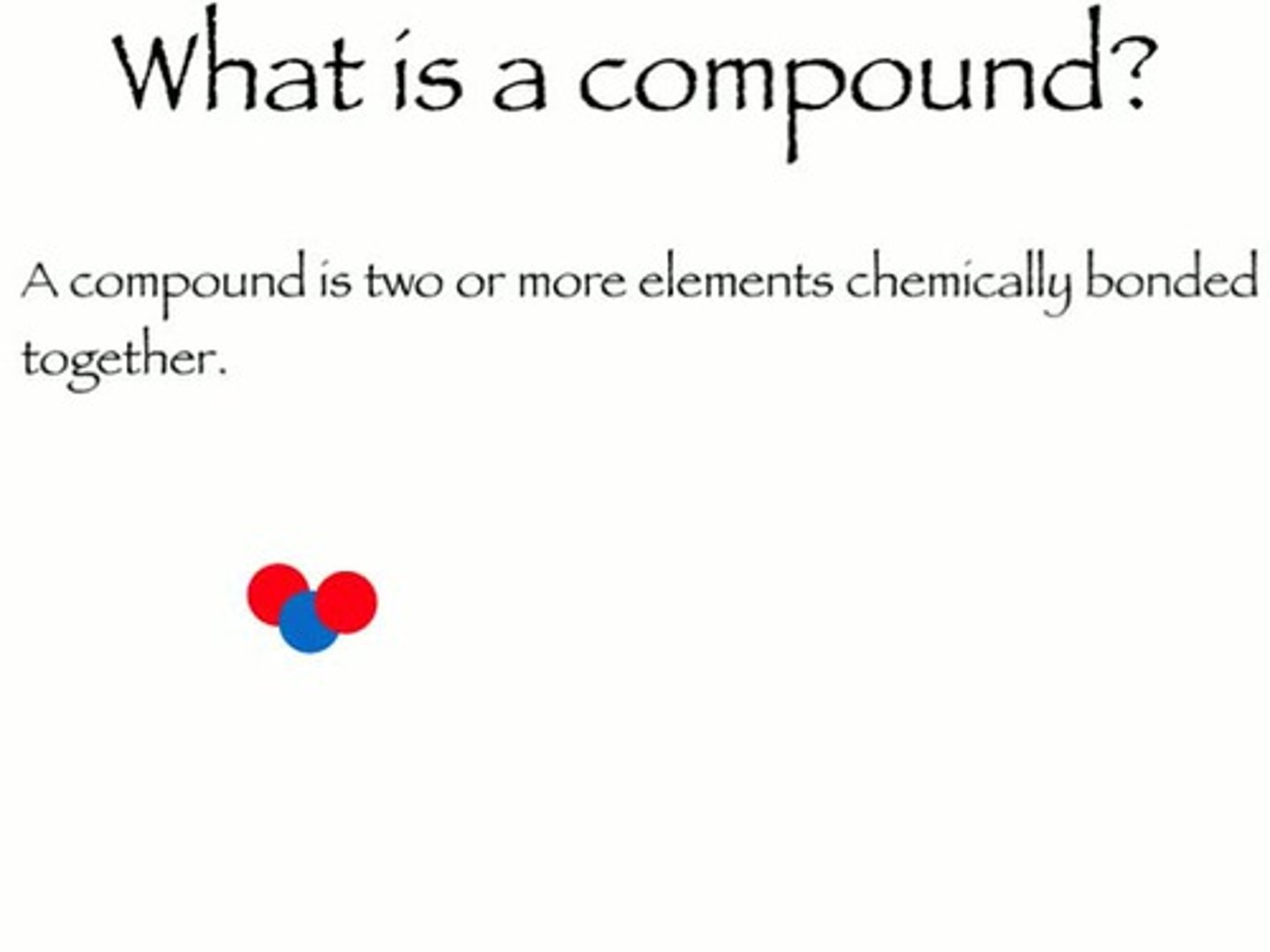
compound
a pure substance with unique properties formed whern two or more different elements combine
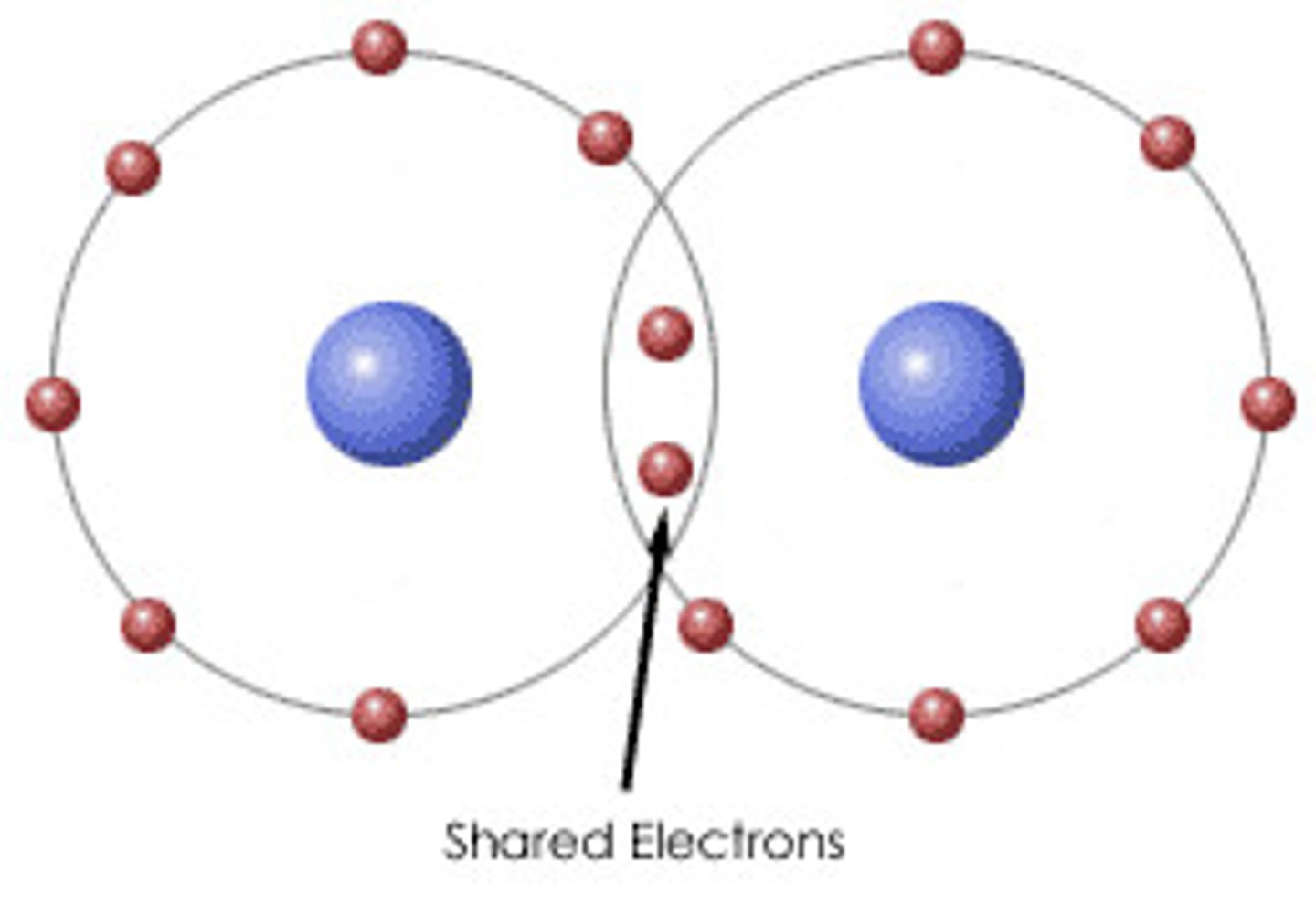
covalent bond
chemical bond which forms when two atoms share electrons
molecule
a compound in which the atoms are held together by covalent bonds
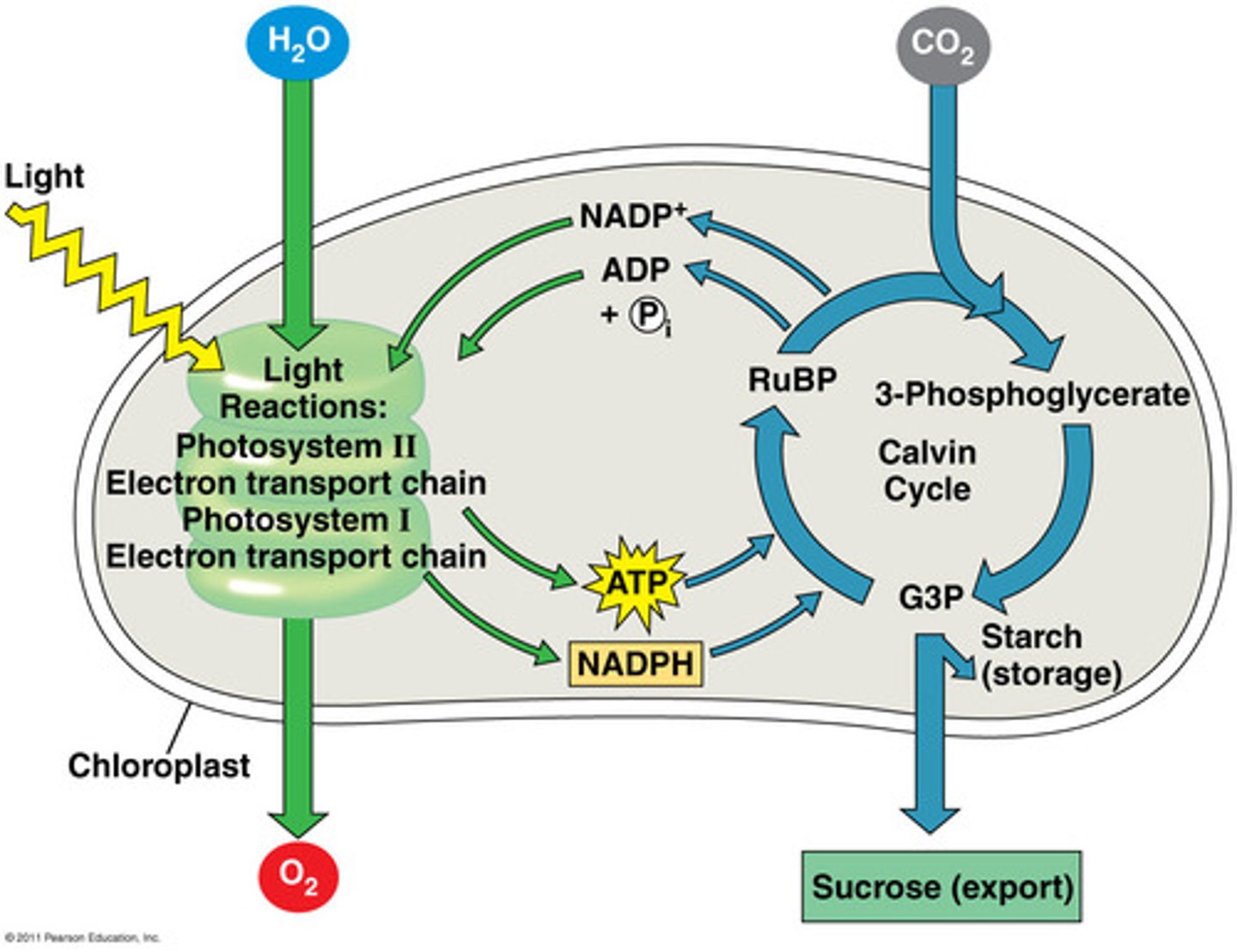
ion
atom that is negatively or positively charged because it has lost or gained one or more electrons
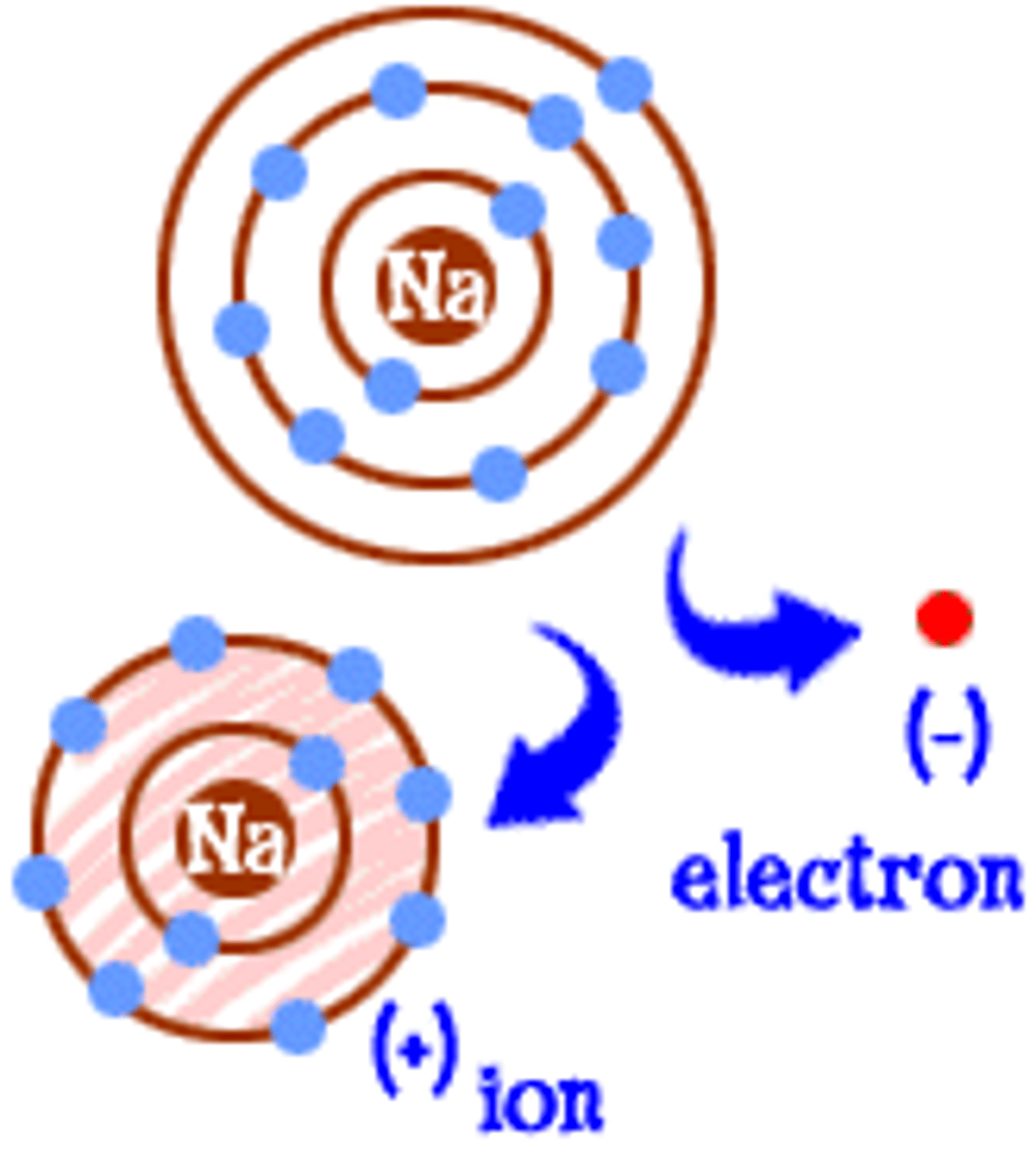
ionic bond
an electrical attraction between two oppositely charged atoms or groups of atoms
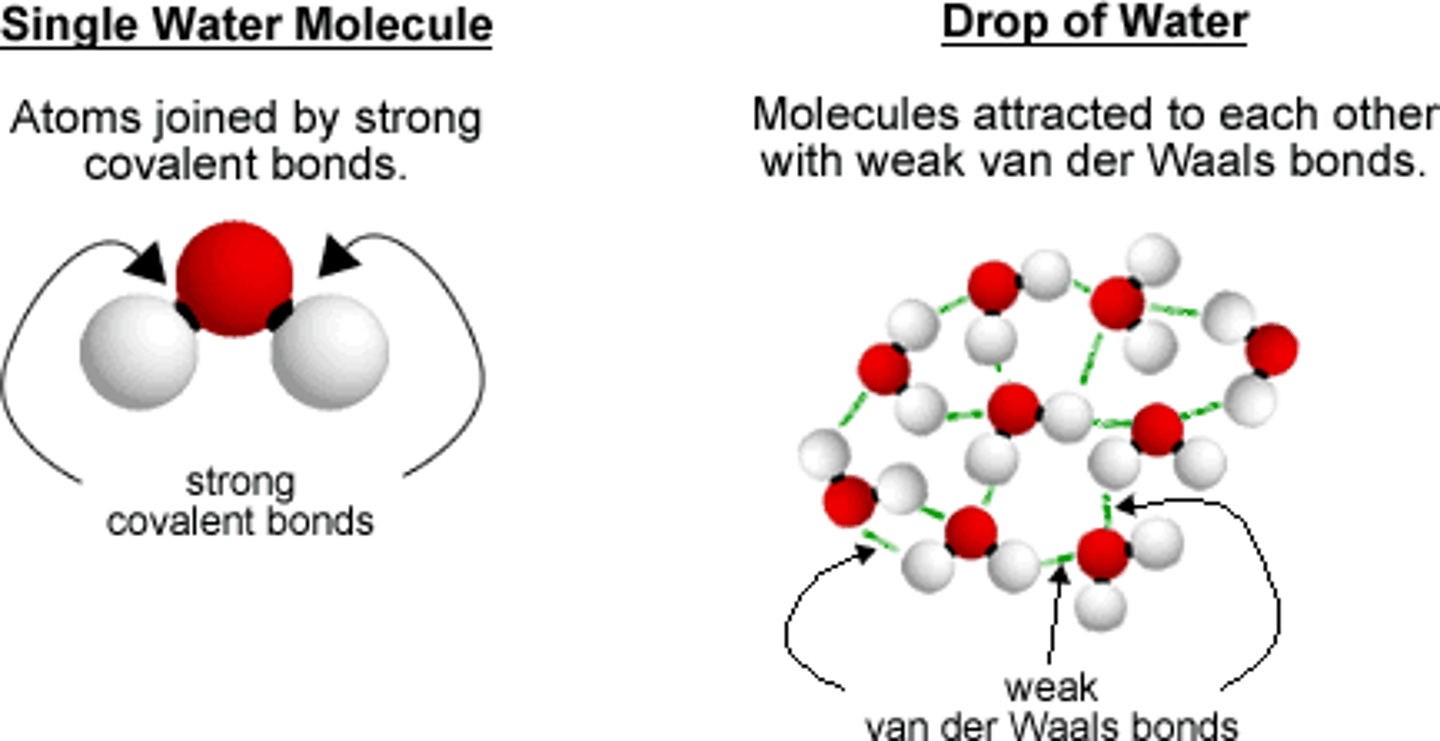
van der Waals forces
the attractive force between the positive and negative regions of different molecules
chemical reaction
energy requiring process by which atoms or groups of atoms are changed into different substances
reactants
substance that exists before a chemical reaction starts
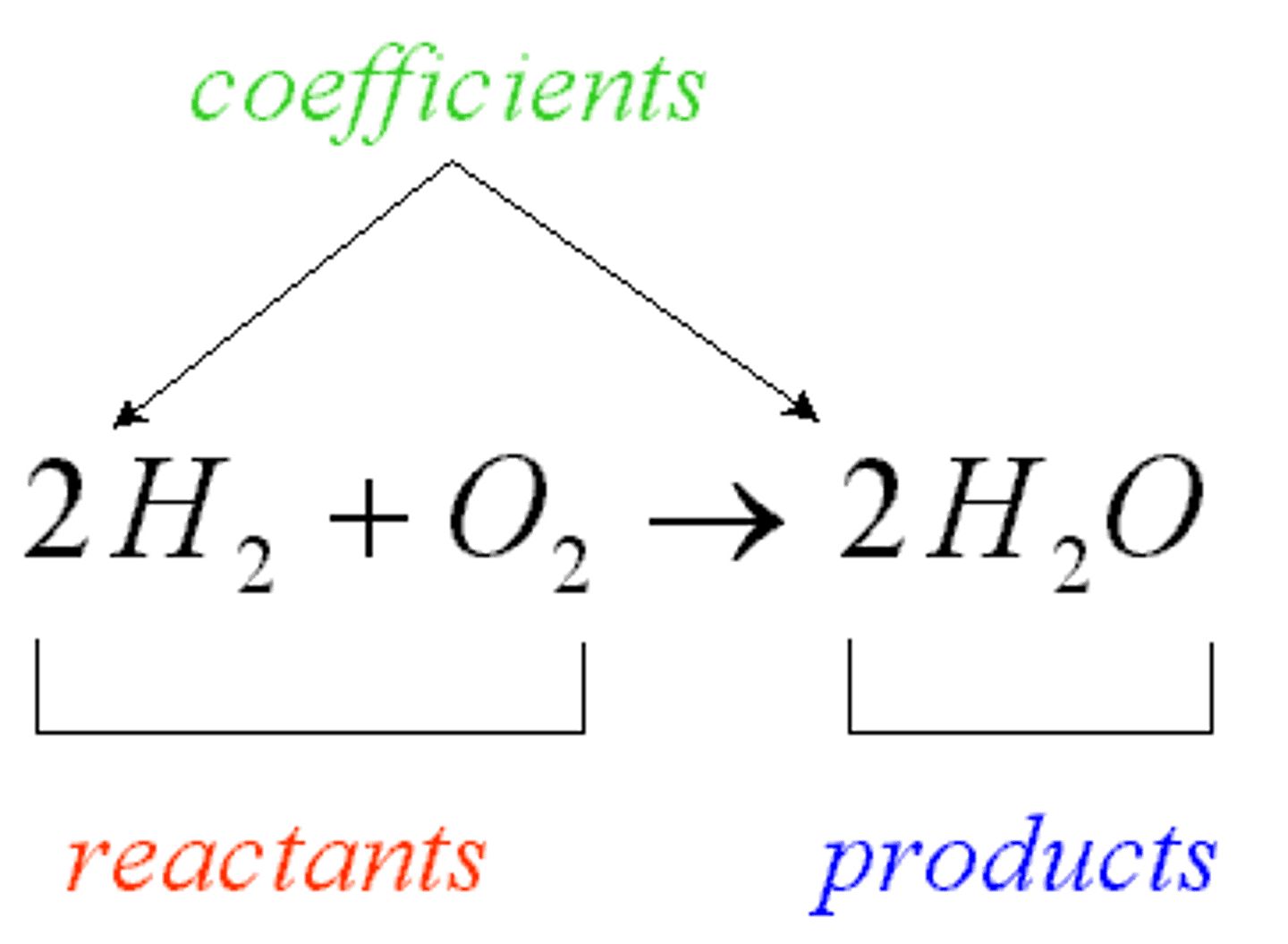
products
the substances formed during the chemical reaction
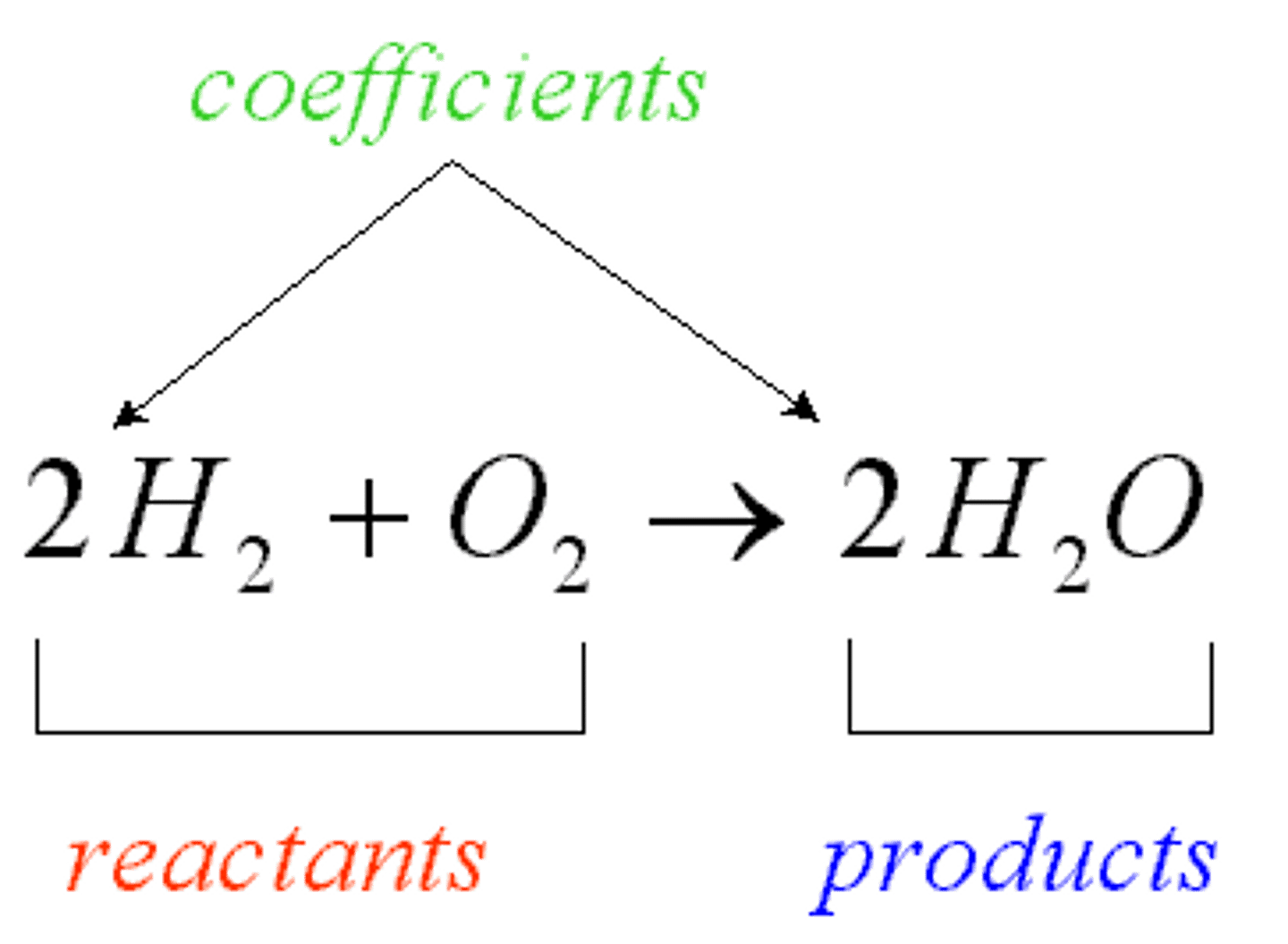
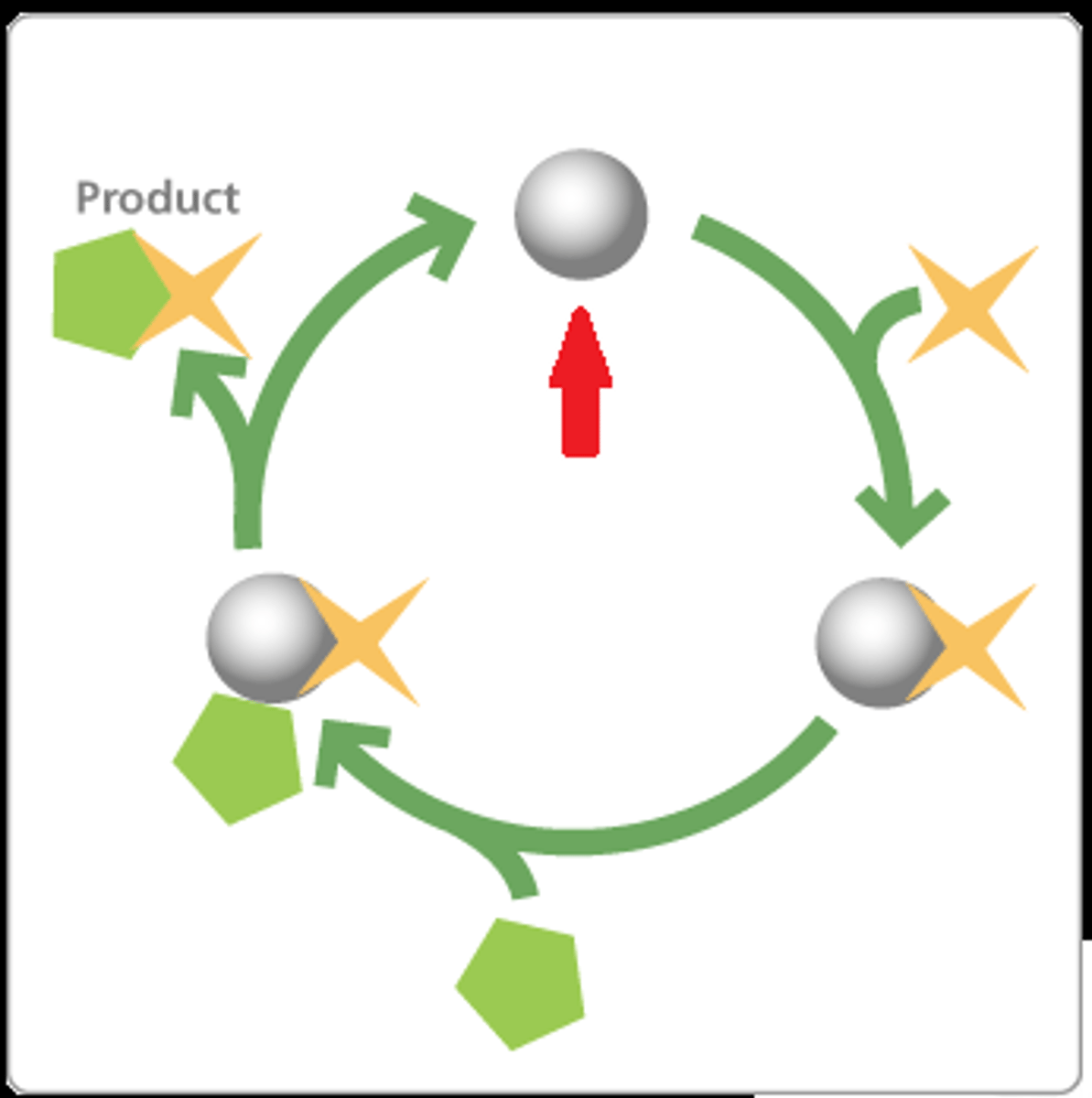
activation energy
the minimum amount of energy needed for the reactants to form products in a chemical reaction
catalyst
substance that speeds up a chemical reaction by reducing the needed amount of activation energy
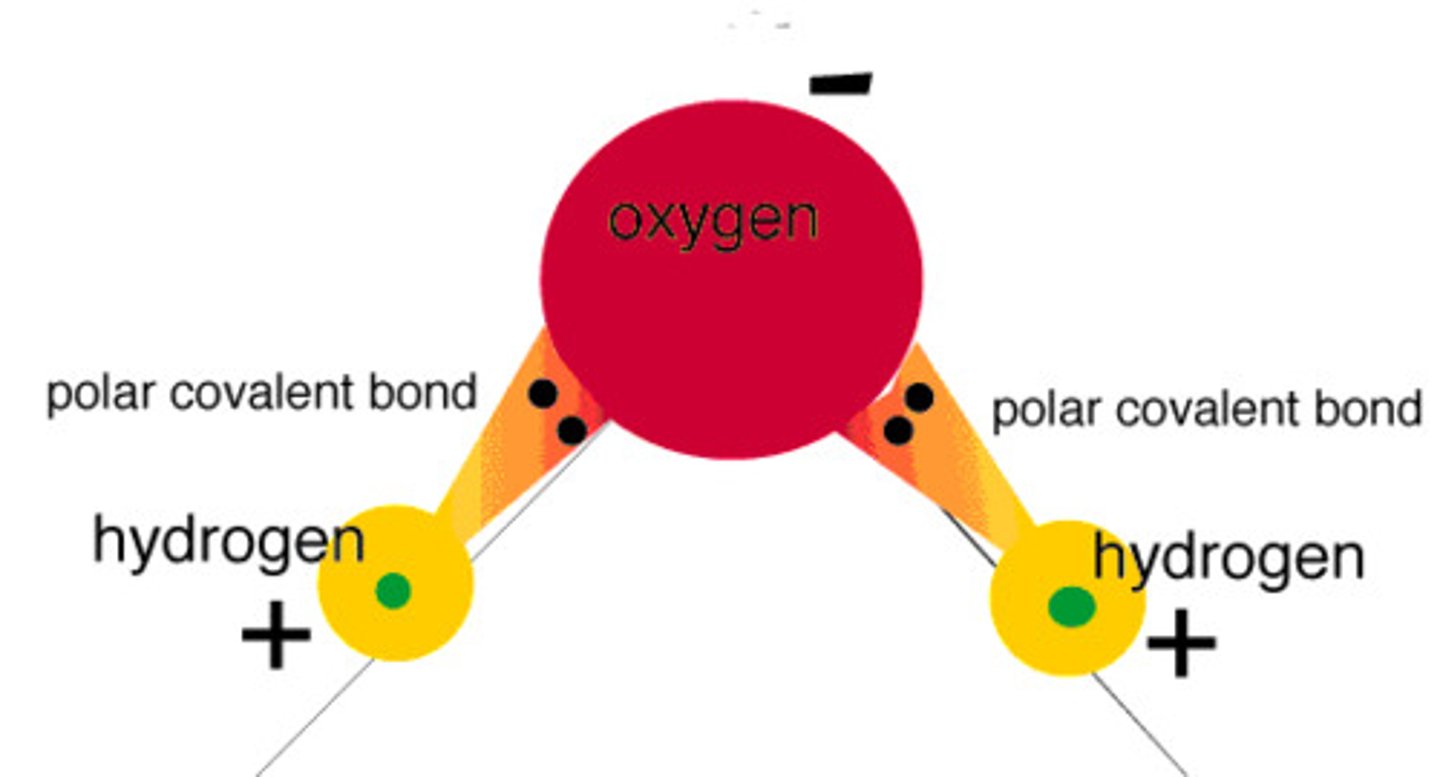
polar molecules
molecule with oppositely charged regions
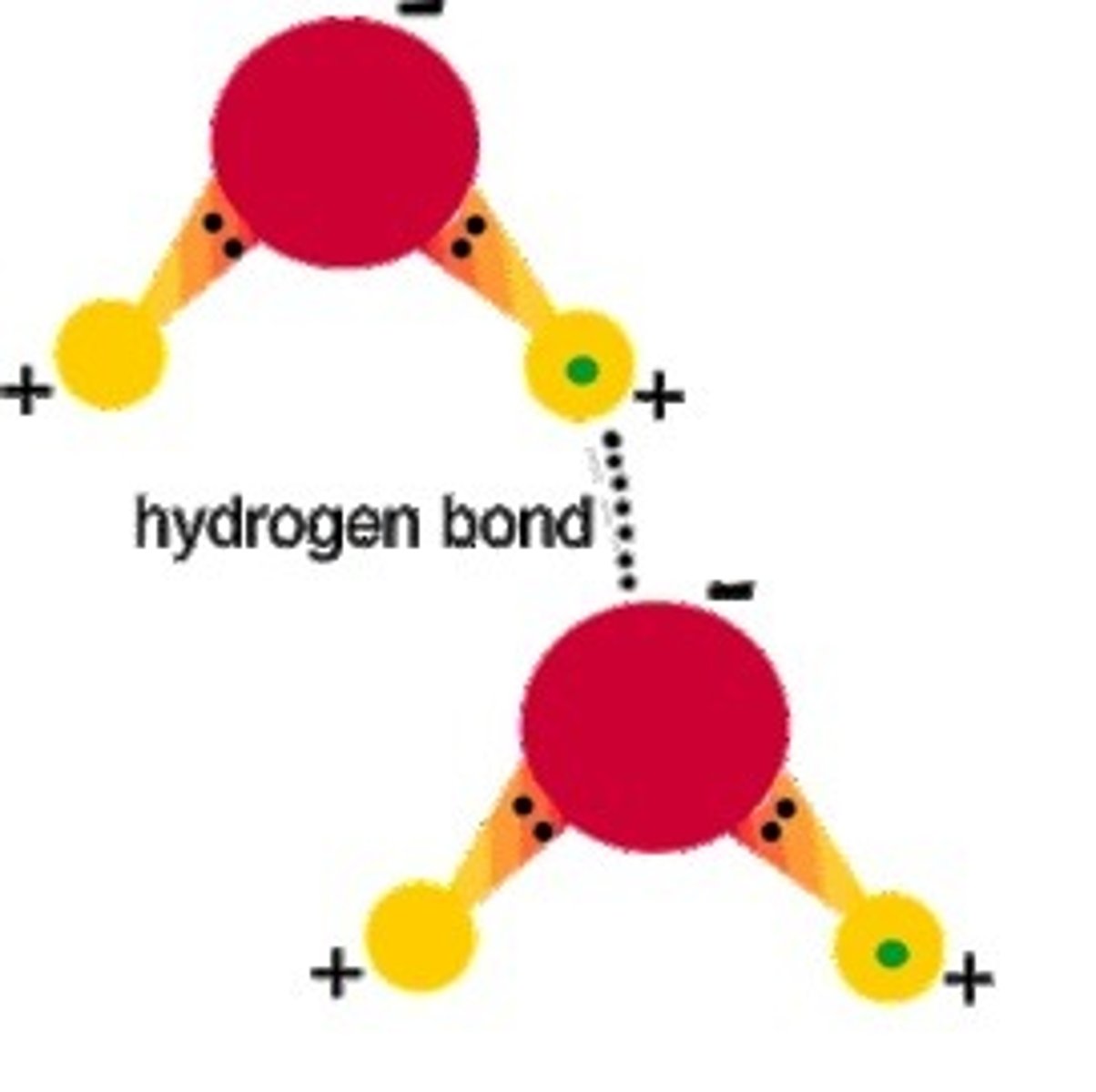
hydrogen bond
weak electrostatic bond formed by the attraction of opposite charges between a hydrogen atom and oxygen, flourine, or nitrogen atom
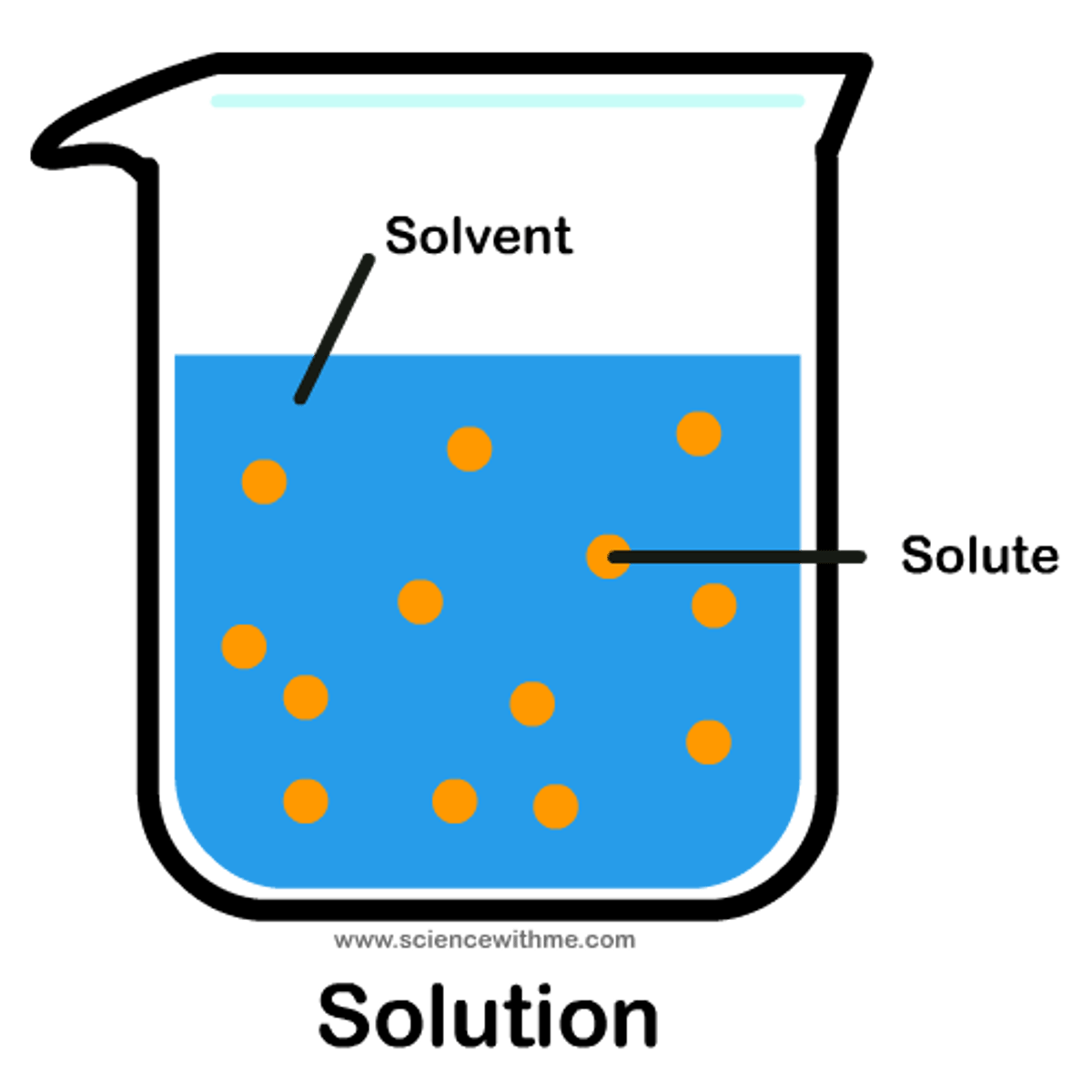
solution
a homogeneous mixture formed when a substance is dissolved in a another substance
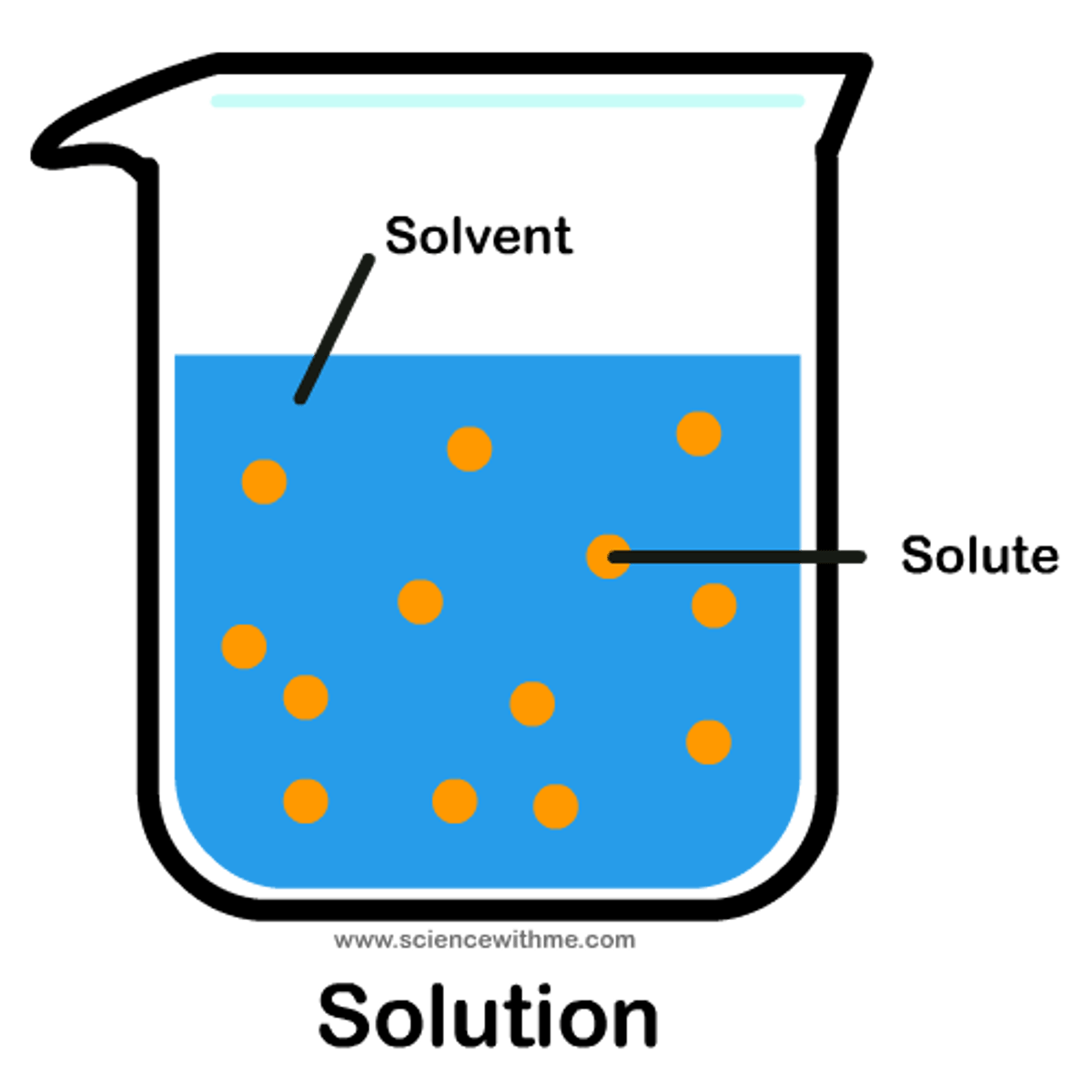
solvent
the substance in which another substance is dissolved
solute
the substance that is dissolved in the solvent
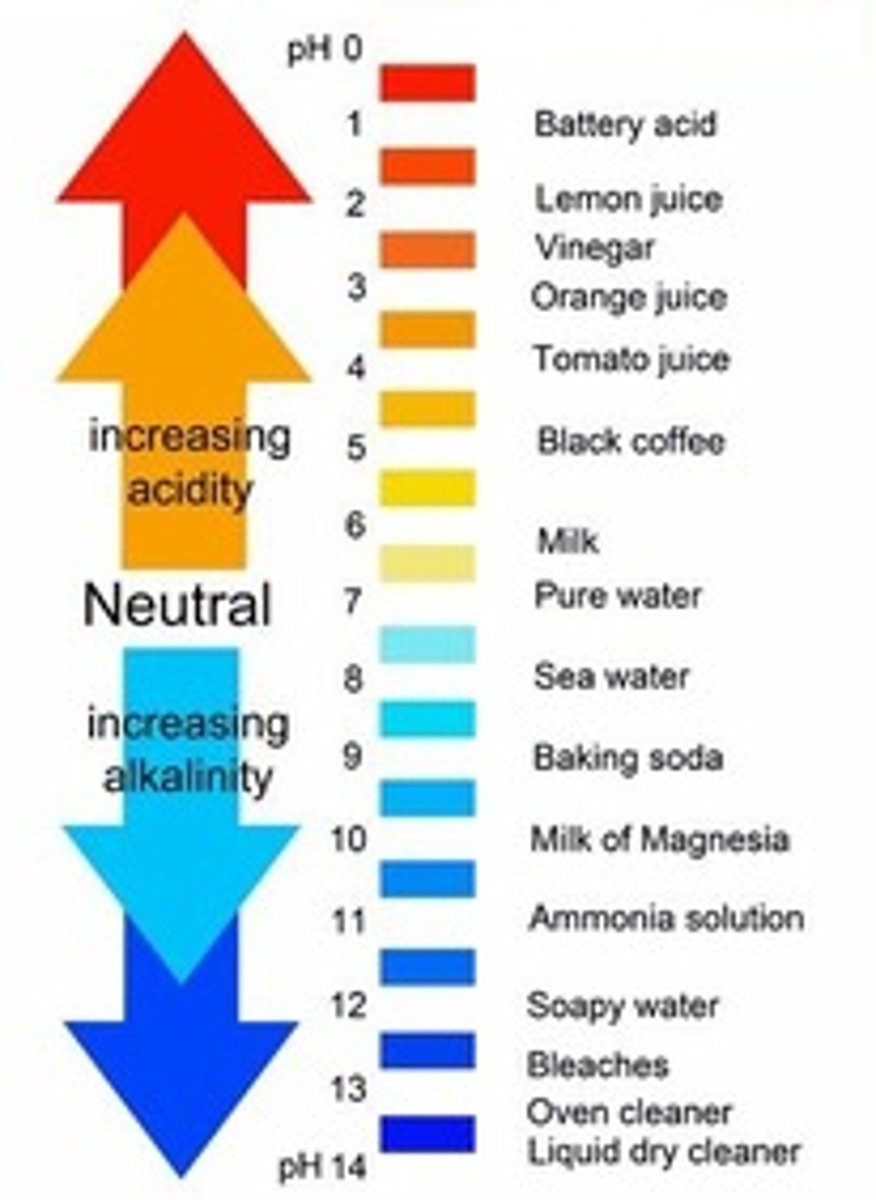
pH
the measure of the concentration of hydrogen ions present in a solution
acids
substances that release hydrogen ions when dissolved in water
buffers
mixtures that can react with acids or based to keep pH within a particular range
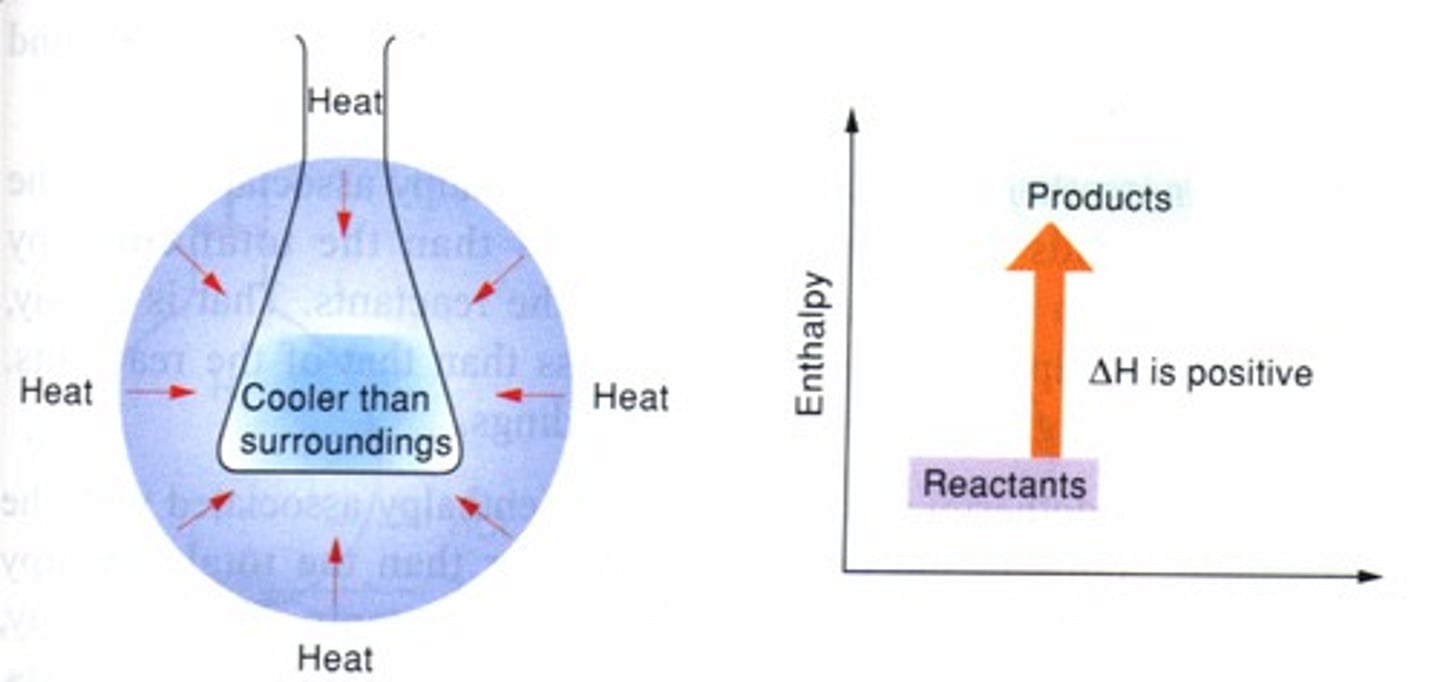
exothermic
Chemical Reaction in which energy is primarily given off in the form of heat
endothermic
(of a chemical reaction or compound) occurring or formed with absorption of heat
0-7
pH of acids
7-14
pH of bases
7
neutral pH
Macromolecules
carbohydrates, lipids, proteins, nucleic acids
carbohydrates
immediate energy and structural support
lipids
Store energy and provide barriers
proteins
Transport substances, speed reactions, support and make hormones.
nucleic Acids
store and transmit genetic information
monosaccharide
simple sugar molecule
disaccharide
A double sugar, consisting of two monosaccharides
polysaccharide
large macromolecule formed from monosaccharides
lipids
fats and oils
saturated fat
A lipid made from fatty acids that have no double bonds between carbon atoms
unsaturated fat
A lipid made from fatty acids that have at least one double bond between carbon atoms.
Amino Acids
monomers of proteins
Nucleotides
make up a nucleic acid that consist of a pentose, phosphate group, and nitrogenous base.
DNA
deoxyribonucleic acid
RNA
ribonucleic acid
What distinguishes organic compounds from inorganic compounds?
Organic compounds contain multiple carbon atoms bonded to each other, while inorganic compounds do not contain carbon atoms, with few exceptions like CO2.
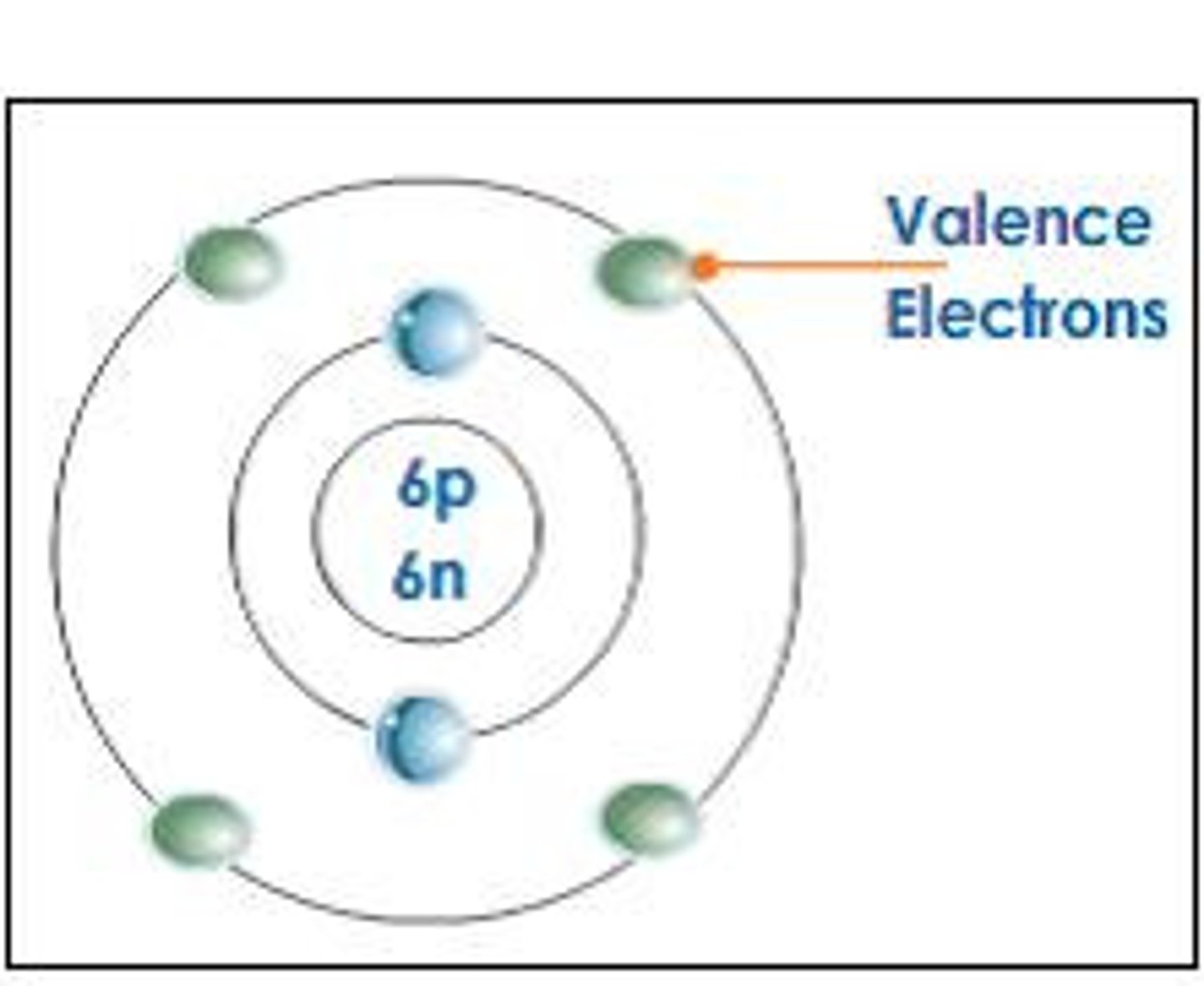
Why is carbon considered an ideal building block for organic molecules?
Carbon has 4 valence electrons, allowing it to form covalent bonds with other atoms, resulting in diverse structures like chains and rings.
What are functional groups in organic chemistry?
Functional groups are groups of atoms attached to carbon skeletons that give molecules specific shapes and are directly involved in chemical reactions.
What is a macromolecule?
A macromolecule is a giant molecule formed by the joining of smaller molecules, typically through polymerization.
What is polymerization?
Polymerization is a reaction in which monomers are joined together to form polymers, often through condensation reactions.
What is the process of condensation in macromolecule formation?
Condensation is when two molecules are covalently bonded through the loss of a water molecule, requiring energy and an enzyme.
What is hydrolysis?
Hydrolysis is the process of breaking down polymers into monomers using water, essentially the reverse of condensation.
What is the general formula for carbohydrates?
Carbohydrates are made up of carbon, hydrogen, and oxygen in a 1:2:1 ratio, represented as CxHyOz.
What are monosaccharides?
Monosaccharides are simple sugars that consist of one sugar molecule and are the monomers of carbohydrates.
What are disaccharides?
Disaccharides are carbohydrates made of two simple sugars covalently bonded together through a condensation reaction.
What are polysaccharides?
Polysaccharides are carbohydrates made up of three or more monosaccharides linked together.
Name three common polysaccharides.
Glycogen, cellulose, and starch.
What are lipids?
Lipids are large, nonpolar organic molecules, including fats, waxes, and oils, that are not soluble in water.
What are the two types of fatty acids?
Saturated fatty acids, which have no double bonds, and unsaturated fatty acids, which have at least one double bond.
What are triglycerides?
Triglycerides are a type of lipid formed from glycerol and three fatty acids.
What are proteins made of?
Proteins are macromolecules made up of carbon, oxygen, hydrogen, and nitrogen, with amino acids as their monomers.
What is the basic structure of an amino acid?
An amino acid consists of a central carbon, a hydrogen atom, an amino group, a carboxyl group, and a unique 'R' group.
What is a dipeptide?
A dipeptide is formed when two amino acids are linked together by a condensation reaction.
What role do enzymes play in biological reactions?
Enzymes are proteins that speed up chemical reactions without being consumed in the process.
What are nucleic acids?
Nucleic acids are macromolecules that contain carbon, hydrogen, oxygen, nitrogen, and phosphorus, and include DNA and RNA.
What is the function of DNA?
DNA stores genetic information that determines the characteristics of an organism and directs cell activities.
What is the role of RNA?
RNA transfers information from DNA in the nucleus to ribosomes in the cytoplasm for protein synthesis.
What is the enzyme-substrate complex?
The enzyme-substrate complex is formed when a substrate molecule binds to the active site of an enzyme.
What are the two models of enzyme action?
The Lock and Key model and the Induced Fit model.
How do heat and pH affect enzymes?
Heat and pH can affect the shape and function of enzymes, potentially denaturing them and altering their activity.
Matter
Anything that occupies space and has mass.
Mass
A measure of the amount of matter in an object.
Weight
The force produced by gravity acting on an object.
Element
Pure substance composed of all the same type of atom.
Atom
The smallest particle of an element that retains all of the properties of that element.
Protons
Positively charged subatomic particles found in the nucleus of an atom.
Neutrons
Subatomic particles that have no charge.
Atomic Number
The number of protons contained in the nucleus of an atom.
Mass Number
The combined total of protons and neutrons in the nucleus of an atom.
Electrons
Small, negatively charged subatomic particles that move about the nucleus at very high speeds and are located in orbitals.
Orbital
The three-dimensional region around the nucleus of an atom that indicates the probable location of an electron.
Isotopes
Atoms of the same element that have different numbers of neutrons.
Compound
A substance composed of atoms of two or more elements in a fixed proportion.
Chemical Bonds
The attractive forces that hold atoms together in compounds.
Covalent Bond
A bond that is formed when two atoms share one or more pairs of electrons.
Molecule
The simplest unit of a compound that retains all of the properties of that substance.
Ion
An atom or molecule that has lost or gained one or more electrons resulting in an atom or molecule with an electrical charge.
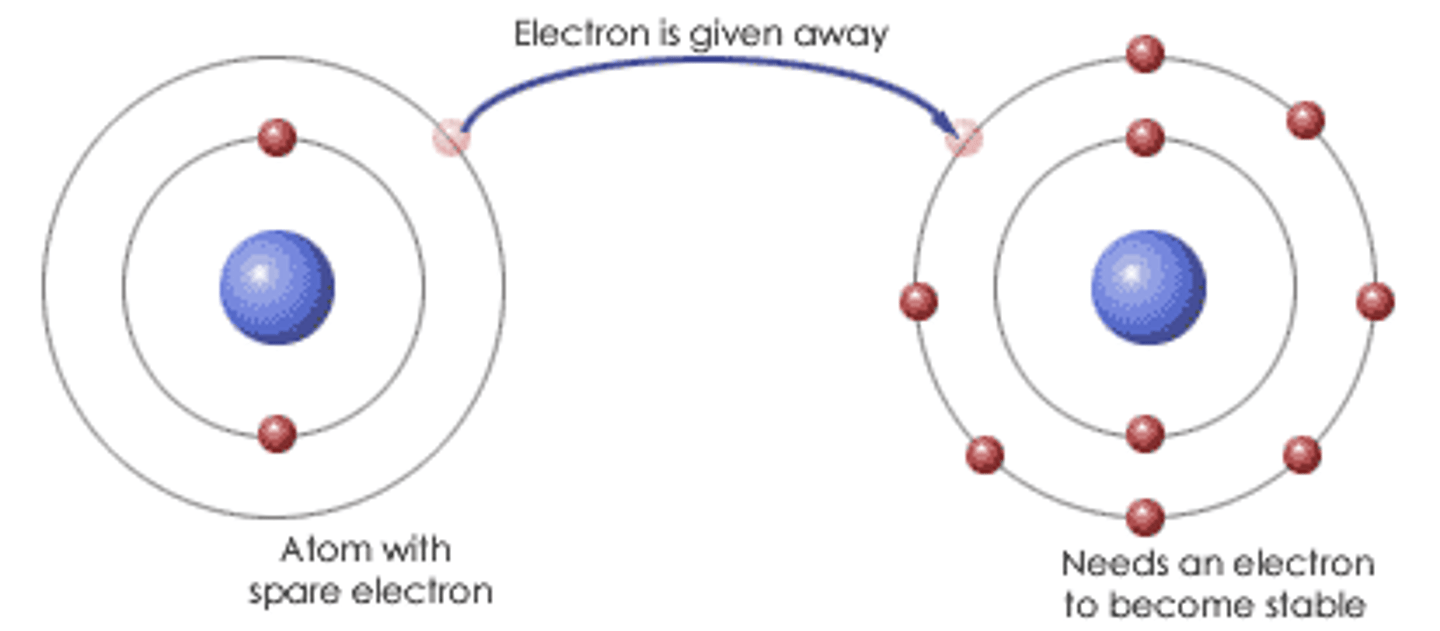
Ionic Bond
A bond that is formed when one or more electrons are transferred from one atom to another.
Energy
The ability to do work.
Chemical Reaction
A process that changes one set of chemicals into another set of chemicals.
Reactants
The elements or compounds that enter into a chemical reaction.
Products
The elements or compounds produced by a chemical reaction.
Metabolism
The sum total of all of the chemical reactions occurring within a cell.
Activation Energy
The amount of energy required to start a reaction.
Catalyst
Inorganic substance that speeds up a chemical reaction by lowering the amount of activation energy needed for the reaction to occur.
Polar Molecule
A molecule in which electrons are shared unequally between the atoms, resulting in a molecule that has poles. Part of the molecule is negative and part of the molecule is positive.
Hydrogen Bond
A weak molecular bond formed between the hydrogen atom of one molecule and a highly electronegative atom of another molecule.
Cohesion
The attraction between molecules of the same substance.
Adhesion
The attraction between molecules of different substances.
Capillary Action
The tendency of water to rise in a thin tube due to cohesion and adhesion.