Fundamentals of chemisty: Acid and bases and pH
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What is pH?
The measure of the acidity of a solution (pH stands for “power of hydrogen”)
a measure of the activity of dissolved hydrogen ions
Hydrogen ions occur as various of cations including protons and hydronium ions (H3O+) in solution
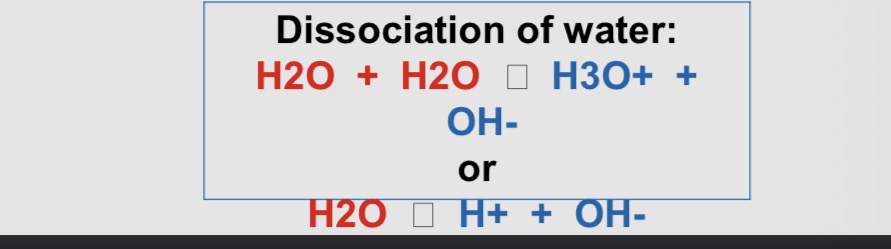
What happens to the conc of hydrogen ions in pure water?
• In pure water at 25 °C the concentration of hydrogen ions
(H+) equals the concentration of hydroxide ions (OH-)
• "neutral" corresponds to a pH level of 7.0
• ACID Solutions are where concentration of [H+] exceeds
that of [OH- ]. They have a pH value lower than 7.0
• BASIC Solutions are where [OH- ]exceeds [H+]. They
have a pH value greater than 7.0
pH of everyday solutions
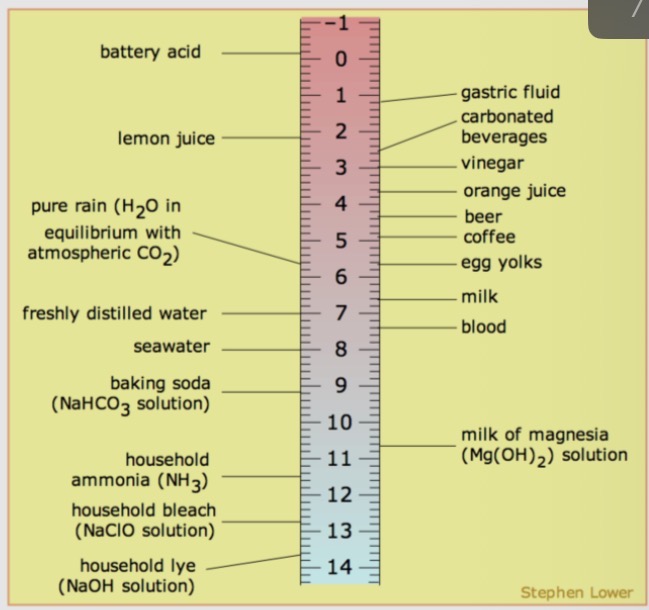
What is low pH?
low pH values solutions with high conc of hydrogen ions
What is high pH?
high pH values solutions with low conc of hydrogen ions
What is pure water?
Pure water has pH of 7.0, other solutions are often described with reference to this value
Acid and bases definitions
Acids solutions that have a pH less than 7 (I.e. more hydrogen ions than water)
Bases a pH greater than 7 (I.e less hydrogen ions than water)
The definition of weak and strong acids OR weak and strong bases does not refer to pH value
It describes how well an acid or bases ionizes in solution
Bronsted-Lowry Theory:
Acid is a proton donor
Bases is proton acceptor
NOTE: this definition is independent of water
What is the pH of a solution defined as?
pH = -log[H3O+]
• pH scale - a scale that indicates the acidity or basic nature of a solution
• Measure of number of H+ ions (or equivalent) in solution
• Ranges from 0 (very acidic) to 14 (very basic)
pH = -log[H+]
A measure of the effective concentration of hydrogen ions (rather than the actual concentration)
In practice hydrogen ions could be shielded/hidden so not available to participate in chemical reactions e.g. inside a protein molecule
Having solutions at the correct pH is critical to living organisms
The case of water
pure water almost 100% molecular (covalently bonded)
Very small amount is ionised (dissociated)
> H20 + H20 → H3O+ + OH-
In pure water at room temperature
[H3O+] = 1 × 10-7 M
[OH-] = 1 × 10-7 Mu
[ ] = mean the concentration of a substance
pH = - log [H3O+]
pH = - (-7)
pH = 7
However, when pure water is exposed to the atmosphere
CO2 will be absorbed and react with water to form carbonic acid (HCO3- and H+) so pH lowered to approx 5.7
pH is a logarithmic scale
pH 7 → 0.0000001 M
pH 6 → 0.0000001M
pH 5 → 0.00001M
pH 4 → 0.0001M
pH 3 → 0.001M
pH 2 → 0.01M
pH 1 → 0.1M
pH 0 → 1M
The difference between each value is 10-fold
What are examples of strong and weak acids?
Strong acids:
• HCl Hydrochloric acid
• HNO3 Nitric acid
• H2SO4 Sulphuric acid
• HI Hydroiodic acid
Weak acids:
• HClO Hypochlorous acid
• HNO2 Nitrous acid
• H2SO3 Sulphurous acid
• HF Hydrofluoric acid
• CH3COOH Ethanoic acid
• H2CO3 Carbonic acid
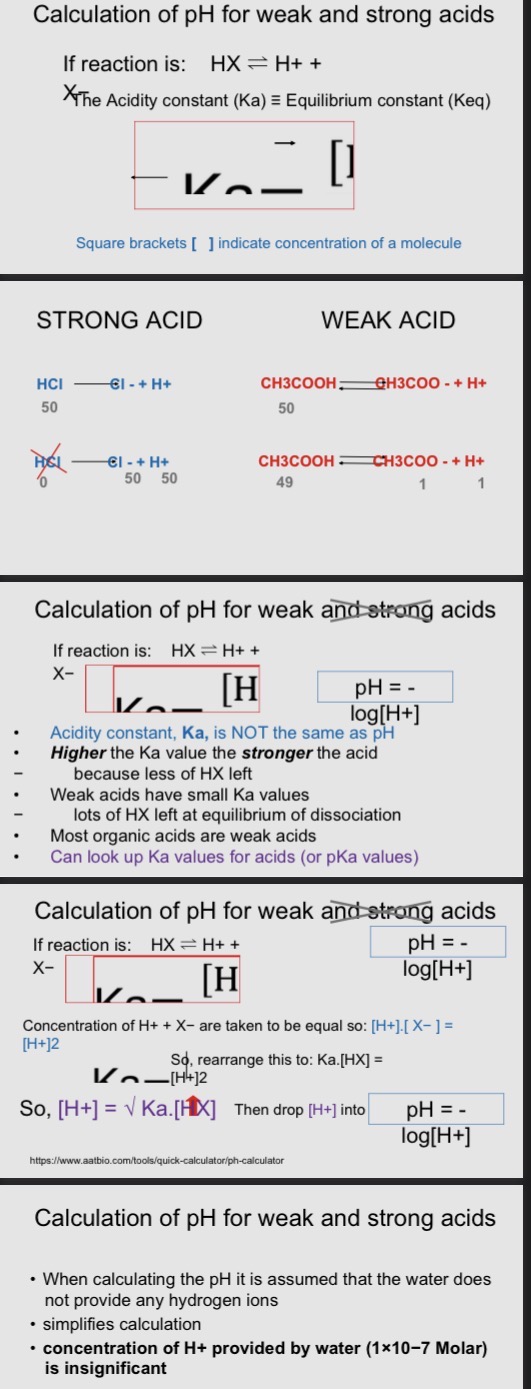
Strong Acids
Strong acid:
HCl + H20 → H+ + Cl-
HCl + H2O → Cl- + H3O+
strong acids; dissociated reaction goes to completion - no unrelated acid remains in solution
Reaction is: HX + H2O → H3O+ + X- usually simplified to: HX → H+ + X-
To calculate pH on;y need to know conc of the acid (HX) present
Weak Acids
Weak acids:
CH3COOH + H+ => CH3COO- + H2O
CH3COOH(aq) + H2O(l) => CH3COO-(aq) + H3O+(aq)
> H3O+ is an hydronium ion
Weak acids: dissociation reaction does not go to completion - unrequited acid remains in solution
An equilibrium is reached between the hydrogen ions and the conjugate base
Reaction is: HX => H+ + X-
For methanoic acid: HCOOH(aq) => H+ + HCOO-
To calculate pH need to know balance of equilibrium reaction (equilibrium constant) - called Aciditiy contact (Ka)
=> is reversible arrows
Bases
• A strong base is a base which hydrolyzes completely, raising the pH of the solution towards 14
• Arrhenius bases are water-soluble and donate hydroxide ions (OH-)
• Alkalis are bases. However, not all bases are alkalis
• Alkalis are Arrhenius bases and hydroxides of the alkali
metals e.g. sodium, potassium
• Strong bases: NaOH, KOH, Ca(OH)2
• Weak bases: NH3 (ammonia)
Bases and pH
pH = -log[H3O+]
• The strong base sodium hydroxide ionizes into hydroxide
and sodium ions in solution:
NaOH → Na+ + OH−
• Pure water dissociates :
2H2O(l) → H3O+(aq) + OH−(aq)
• When these are mixed the H3O+ and OH− ions combine to
form water molecules:
H3O+ + OH− → 2 H2O
• The hydroxide ion ‘removes’ the available hydronium /
hydrogen ions, lowering their concentration, so pH value
goes up
![<p><strong>pH = -log[H3O+]</strong></p><p></p><p>• The <strong>strong base </strong>sodium hydroxide ionizes into hydroxide</p><p>and sodium ions <em>in solution</em>:</p><p><strong>NaOH → Na+ + OH−</strong></p><p>• Pure water dissociates :</p><p><strong>2H2O(l) → H3O+(aq) + OH−(aq)</strong></p><p>• When these are mixed the H3O+ and OH− ions combine to</p><p>form water molecules:</p><p><strong>H3O+ + OH− → 2 H2O</strong></p><p>• The hydroxide ion ‘<strong>removes</strong>’ the available hydronium /</p><p>hydrogen ions, lowering their concentration, <strong>so pH value</strong></p><p><strong>goes up</strong></p>](https://knowt-user-attachments.s3.amazonaws.com/5f81d596-ae2d-4953-abd4-10e915588c75.jpg)
Neutralisation
• the reaction between an acid and a base
• will produce a salt and neutralized base
• hydrochloric acid and sodium hydroxide form sodium
chloride and water:
• HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq)
H+ (aq) + OH- (aq) => H2O (l)
• Neutralization will not always give a solution with pH 7.0
– Only if similar strength acids and bases reacted
– a strong acid and a weak base will give a weakly acidic salt and vice-versa
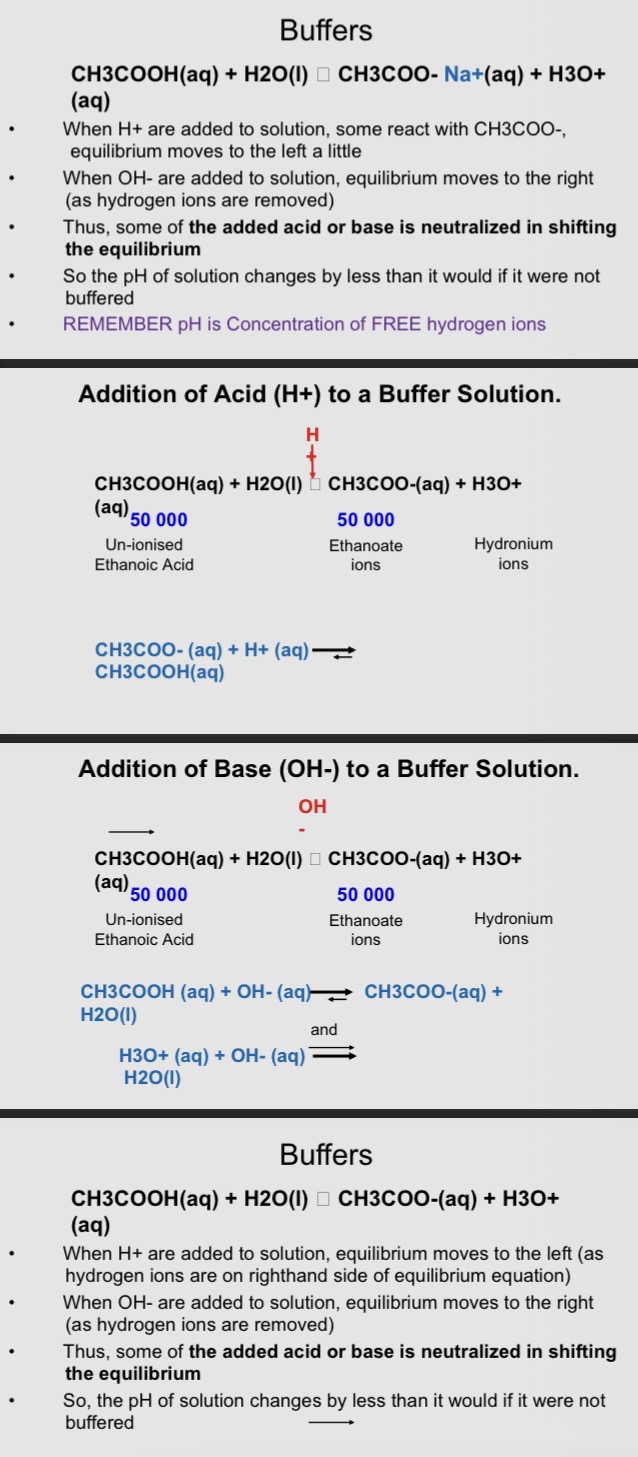
Buffers
• Buffer solution - solution which resists large changes in pH when small volumes of acids or bases are added.
• Buffers consist of either:
• a weak acid and its salt or a weak base and its salt
• Buffers are essential to life and biochemical reaction
• Buffer solution: an aqueous solution consisting of a
mixture of a weak acid and its conjugate base or a weak
base and its conjugate acid
• Buffers mean that pH of solution changes very little when a
small amount of acid or base is added to it
• Buffer solutions are used as a means of keeping pH at a
nearly constant value in biochemical applications
• Buffers are in cells and blood to maintain physiological pH
– E.g. blood plasma is at pH 7.4 via bicarbonate-carbonic acid
Henderson-Hasselbalch equation
Useful for estimating the pH of a buffer solution and finding the equilibrium pH in acid-base reactions
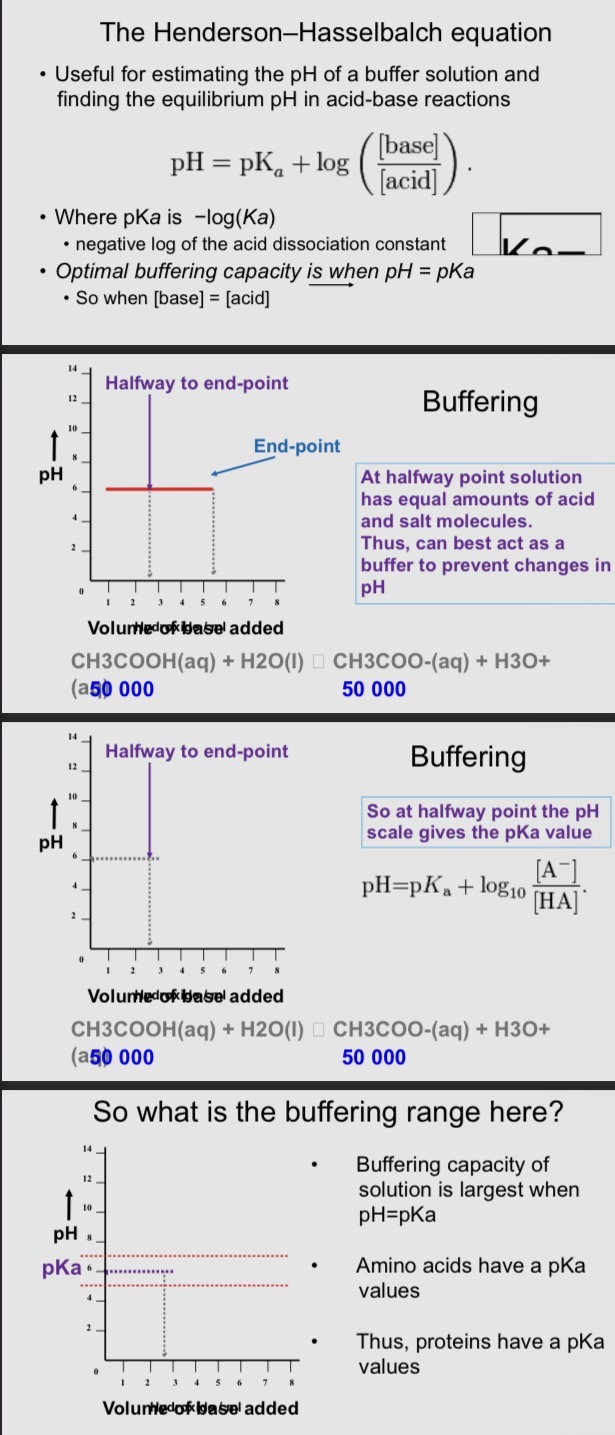
Buffers in biology
• Resistance to changes in pH make buffer solutions
essential for many biochemical processes
• An ideal buffer for a particular pH has a pKa equal to that
pH. Thus, the solution has maximum buffer capacity
• Buffer solutions are necessary to keep correct pH for
enzymes to work - many enzymes work only under very
precise conditions
• A buffer of carbonic acid (H2CO3) and bicarbonate
(HCO3−) is present in blood plasma - maintains a pH
between 7.35 and 7.45