Intermolecular forces/Liquid/Gas phase/Kinetics
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Hydrogen bonding (strongest)
F-H, O-H, or N-H bond required in a pure substance
F, O, or N to hydrogen bond with H2O
dipole-dipole forces (2nd strongest)
intermolecular force for polar molecules
London dispersion forces (Van der Waals)
A temporary or transient dipole
All molecules have these and the greater the weight and surface area, the greater the London dispersion forces
Higher intermolecular forces lead to —-
Higher boiling point
higher heat of vaporization
higher viscosity
higher surface tension
lower vapor pressure
Ideal Gas Law
PV=nRT
Boyle’s Law
P = 1/V
Charle’s Law
V = T
Avogadro’s Law
V = n
combined gas law
PV/nT = PV/nT
catalyst
Speeds up a reaction by lowering the activation energy, by providing an alternate mechanism for the rxn to occur
NOT consumed in a rxn
does NOT shift equilibrium
kinetic product
lower activation energy
favored at low temperatures
thermodynamic product
Overall lower energy product
favored at higher temperatures
reaction order zero
(A) = (A)0 - kt
t1/2 = (A)0/2k — the less reactant the shorter it will take
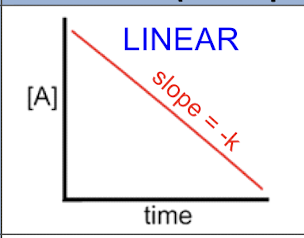
1st order rxn
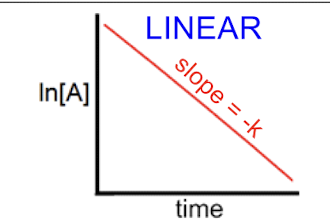
ln(A) = ln(A)0 -kt
t1/2= 0.693/k — does not matter how much you have
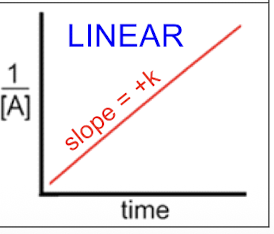
2nd order rxn
1/(A) = 1/(A)0 + kt
t1/2 = 1/k(A)0 — the less of a reactant the longer it will take
K >> 1
products favored at equilbrium
K << 1
reactants favored at equilibrium
Q < K
shift right
Q > K
shift left
Q = K
at equilibrium