Virtual Vista science 20 Unit A chapter 1-2 conclusion
1/112
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
113 Terms
Solutions
homogeneous mixture (two or more substances distributed) where the solute and solvent are not visible. Often liquid, but can be solids. Gasses are liquid in a different form.
Solute
a substance which bonds are broken by a solvent; minor part of solution, and what gets dissolved.
Solvent
greater proportion in the mixture, breaks down solutes bonds.
Physical properties
conductivity, colour, hardness, flexibility
Chemical properties
how composition of matter changes, or how it reacts chemically
Element
pure substance that cannot be broken down simpler by chemical means
Atom
smallest part of an element that has all of its properties
Proton
positively charged particle in nucleus of an atom
Neutron
neutral particle in nucleus of an atom
Electron
negatively charged particle outside atoms nucleus
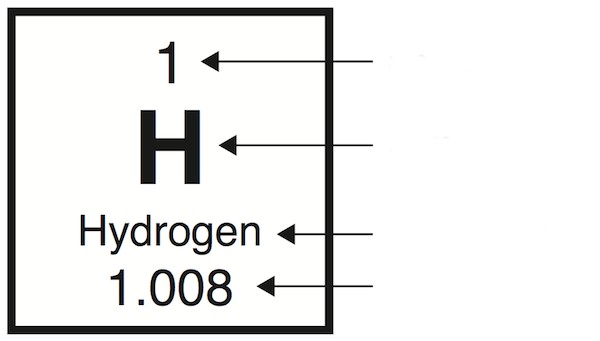
Atomic number
the number of protons inside atom’s nucleus
Atomic mass
average mass of an elements particles; protons, neutrons (not electrons, barely hold weight)
Mass number
The total count of protons and neutrons in a specific isotope of an element (because isotopes of an element have a different amount of neutrons than the base element) . Since atomic mass is closely related to the mass number, you subtract atomic mass by atomic number, and round up for the most common neutron count
How to find the number of neutrons in an atom?
For an exact count of neutrons of an isotope: subtract the mass number (sum of protons + neutrons) by the atomic number (protons)
—But because the mass number is of the most common isotope, and often close to the rounded atomic mass (which reflects the average total weight of all isotopes)—
For a rough estimate of the most common isotope: round atomic mass (total weight of atom) to nearest whole number and subtract by the atomic number (protons)
Do protons and neutrons compose most of an atoms volume?
While its true they compose most of an atoms mass, they are not most of the volume, as they are located in the nucleus, a tiny spot in the middle of the atom.
Electron cloud
A term used to describe the space outside the nucleus within the atom where electrons reside. The term "cloud" comes from the rapid, unpredictable motions of electrons within orbital paths around the nucleus.
Why are electrons bound to the nucleus?
Oppositely charged objects are attracted to each other
How do atoms not repel each other if they both have a surrounding negatively charged field
Although they may initially repel, the large concentration of positive charge in their nuclei prove to be a stronger attracting force, both pulling the other atoms electron cloud simultaneously.
Quarks
the extremely tiny particles that make up protons and neutrons
Energy level
specific region outside of nucleus available for electrons. Only a certain amount of electrons are able to occupy an energy level. The level closet to the nucleus have the least amount of energy, and are filled first, with the others following in ascending order.
What is all matter made up of ?
A large number of associated atoms. The proton, nucleus, and electrons of an atom determines how it bonds with other atoms. These bonds determine physical and chemical properties of their respective substance. The structure of atoms interacting within a substance determine if it shines, is soft/rigid/ or can conduct energy.
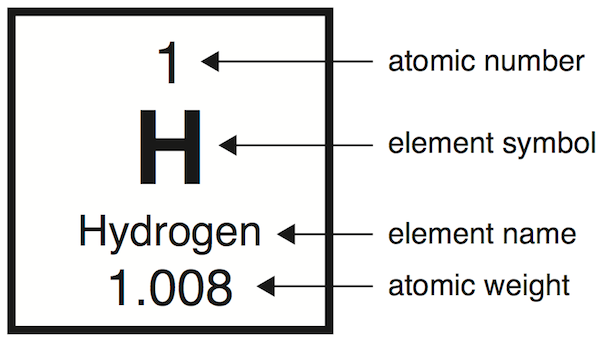
Label the terms
electrons are equal to the number of protons (atomic #) in a stable atom
+1 positive and -1 negative contribute to a neutral charge: 0.
How many electrons fill each energy level?
from the 3 energy levels of an atom, 2 maximum fit in the first layer, and 8 in the second and third layers.
Valence electrons
Electrons on the outermost energy level
What is the transfer of electrons when a metal is in the equation?
Metals usually lose and become cations, while nonmetals “steal” the electron to become negatively charged anions.
ion
a charged atom. Anytime an atom is not balanced in charge— no longer stable— it becomes an ion. Non-metal takes on suffix ‘-ide’. Unless, the non-metal is a molecule with a whole (-) or (+) charge (polyatomic ions— diff from atoms bonding w/ each other), it takes on ‘-ate’.
ex: oxygen gains 2 electrons: oxide ion
phosphate (phosphorus and oxygen molecule with negative charge) bonds with aluminum: aluminum phosphate
tl/dr:
non-metal ion: ‘ide’
non-mental polyatomic (charged molecule): ‘ate’
Cation
positively charged ion
Anion
negatively charged ion
What are the number of valence electrons and tendencies for Alkali metals, Halogens, and noble gasses?
Alkali metals (group 1): 1 valence electron; highly reactive, as they only need to lose one electron to form a stable bond
Halogens (group 12, reactive non-metals): 7 valence electrons; highly reactive— only need to gain 1 to form a stable bond
Noble gasses (group 8): 8 valance electrons (helium has 2); inert, rarely form compounds due to a full valence shell.
Why do elements in the same group share similar chemical and physical properties?
Because they tend to gain, lose, or share electrons in a predictable manner— due to the same amount of valence electrons.
And because bonding behavior affects melting point, boiling point, conductivity, and solubility.
Ex:
Alkali metals are all soft, low-density metals with low melting points due to their weak metallic bonding (tending to only form a single electron bond).
What are the characteristics of the 3 groups?
Alkali: soft, highly reactive metals that form strong bonds w/ water
Halogens: non-metals who have strong attraction for outside electrons, form salts w/ metals
Noble gasses: gasses w/ low reactivity
.
.
Each group follows similar periodic trends, such as increasing in atomic weight and reactivity as you go down the column (except noble gasses, remain mainly unreactive.)
Difference between Bohr and the Electron Dot model of an atom?
Bohr: shows all electron levels
Electron dot: only shows the valence electrons
What is the importance of the valence shell
When two atoms are in proximity, both nuclei’s positive charge attracts the (-) charged electron clouds. The outer-shell electrons are the significant electrons in this process. How an electron loses or gains (bonds) is determined by the valance. Atoms with similar #’s of valence electrons have similar bonding properties.
Why is reactivity gauged by the valence shell electrons?
Atoms crave to be stable (can have balanced charges and not be stable), and to have whichever one of its energy layers is the outermost full. They tend to take the path of least resistance, where if the choice is to either gain 7 or lose 1 to have a full layer, they would lose 1.
i.e atoms closer to a full shell will more readily gain/lose
Note: by losing this electron, the atom is now stable, but it has more protons than electrons giving it a positive charge; cation.
bond
electrostatic forces keeping atoms or ions together. When bonds form, the full compound is neutral and stable.
Covalent bond
Bond between non-metals where two atoms share electrons to fill each others shell. Imagine the energy layers overlapping, and the electron(s) passing around both atoms.
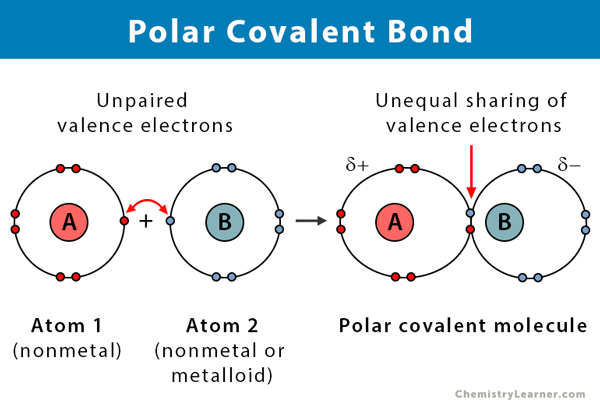
Molecule
Molecule: A group of two or more non-metal atoms held together by covalent bonds, forming a discrete, stable unit.
Molecules have lower melting and boiling points than ionic compounds, are generally flammable, softer, less soluble in water, and do not conduct electricity.
BrINCLHOF twins
non-metals so reactive, they form bonds with themselves. Br2I2N2CL2H2O2F2
Metals tend to lose and non-metals tend to..?
Gain. Non-metals, with the exception of noble gasses, require 1 or more electrons to be stable. Metals require losing 1 or more electrons to be stable.
Molecular compound
A compound composed of identical molecules, typically formed by nonmetals. For example, a bucket of water is just many H₂O molecules coexisting. These molecules are held together by intermolecular forces, not any concrete bonds (inter: between two or more, intra: within).
The molecule “H2O” would be one unit of this structure. Due to the fact there is a single type of molecule within, the structure is a pure substance.
Network covalent solids are the exception to this, where they are, as a whole, made up of covalent bonds—the whole structure is the fundamental unit (debated as molecule).
How do ionic compounds differ from molecular compounds fundamentally?
Unlike molecular compounds, which can be funneled into a single molecule part, ionic compounds only bond as a lattice structure. Ionic compounds, by contrast, do not have standalone “units”—forming as a lattice structure from the instant they are created. They follow a fixed ratio of ions (e.g., Na⁺:Cl⁻ in NaCl), and that ratio simply scales across the entire structure. The formula unit represents the lowest whole-number ratio of ions in this lattice, but it’s not a physically separable particle like a molecule—only a picture of the simplest repeating ratio w/in the structure.
Ionic bonds don’t break into molecule-like fragments. Instead, they dissociate into individual ions. Their identity lies in the crystal lattice—not in any isolated unit.
i.e molecules can chill, and come to form a structure if they so please, but ionic bonds have no base unit and are lattice structure till death
Molecular formula
chemical formula displaying of many atoms of each element a molecule contains
i.e Ethane: C2H6 , which means it has 2 carbon and 6 hydrogen
How do continuous strand molecules work?
C has 4 valence electrons. It must either gain 4 or lose 4 to be stable.
H has 1 valence electron in the first layer. It must gain 1 to be stable.
C and H can share 1 electron to balance out, but while H is stable, carbon must either balance out with more hydrogen or other carbon.
This can create a chain of numerous carbon, which finally ends on a hydrogen
Metallic element, Ionic compound, molecule
metal with metal (metallic)
non-metal with metal (ionic)
non-metal with non-metal (molecular)
Lattice structure
the crystalline shape ionic bonds take on when forming ionic compounds.
bonding within a Metallic element
Ex:
The individual Aluminum atoms (Al) crave stability, so they take the path of least resistance and lose (moreso “shed”) 3 electrons. The atoms are now positive ions, cations, and those electrons are ‘delocalized’ (free moving). The aluminum cations are then held together by their mutual attraction to this "sea" of free electrons.
The metal as a whole remains electrically neutral, since the number of free electrons matches the number of protons.
This is why metals are conductive, the electrons move around freely, and electric current flows as energy jumps from electron to electron within the mobile sea.
Characteristics of ionic compounds?
Generally high melting point, and can conduct electricity when melted or dissolved in water (The dissolved ions float around and allow current to flow)
How do ions attract each other?
ionic bonds involve a transfer of electrons, so one atom becomes (+) and the other (-). The two oppositely charged ions attract each other after becoming charged.
Bond strength
how much energy it takes to break bonds apart
Are ionic bonds or molecular bonds stronger?
Individual covalent bonds between atoms, like C-C, are harder to break apart, although intramolecular bonds are generally easier to break apart.
While ionic bonds on there own are weaker, the lattice structures as a whole are stronger than molecular compounds.
How is melting point related to bond strength?
Heat energy moves atoms/molecules apart. The more energy required (higher heat) means a stronger bond.
Are molecular or ionic compounds stronger at room temp?
At room temp, molecular compound strength varies; gas, liquid, solid, but generally melt lower— weaker intermolecular bonds.
At room temp, all ionic compounds are solid— stronger bonds
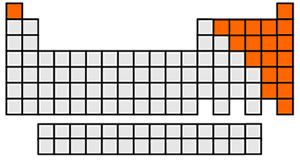
Are there more metals or non-metals on the periodic table?
There are more metals.
The orange blocks are non-metals.
Evidence of chemical change?
Colour change
Change in state; gas or precipitation is produced
odour (gas is produced)
Change in energy (heat expelled or absorbed)
Exothermic reaction (expels heat/light; candle, glowsticks)
Endothermic reaction (absorbs heat; ice pack, cooking egg)
What happens to ionic compounds when broken down in a solution?
The charged ions are made free to form new bonds with other substances in the solution (or just chill).
What is the general process from atom to molecular or ionic compound?
Atom :covalent bond atom → molecule
Molecule : molecule → molecular compound
Ion+ :electrostatic bond Ion- → ionic compound/ lattice structure
What’s so special about water that it is considered the ‘solvent of life’?
an H20 molecule is ‘polar’ meaning it has a negative end and a positive end. It has a partial negative charge (1- oxygen atom), and a partial positive charge (2+ hydrogen atoms) at the bottom of the oxygen.
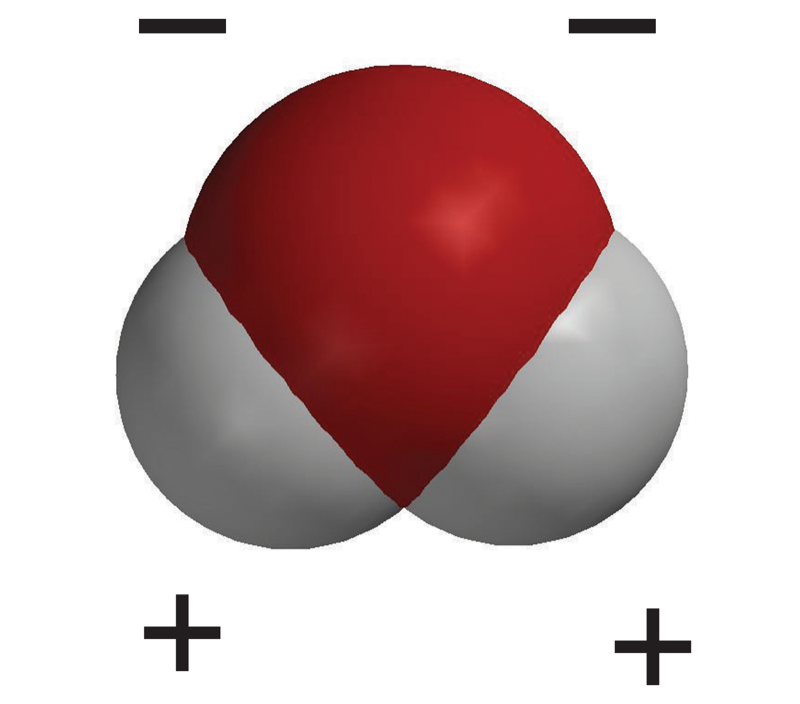
How do water molecules break down an ionic compound?
The oppositely charged ions are held together in a lattice structure.
the positive side of the H2O pulls out the anions
the negative side of the H20 pulls out the cations
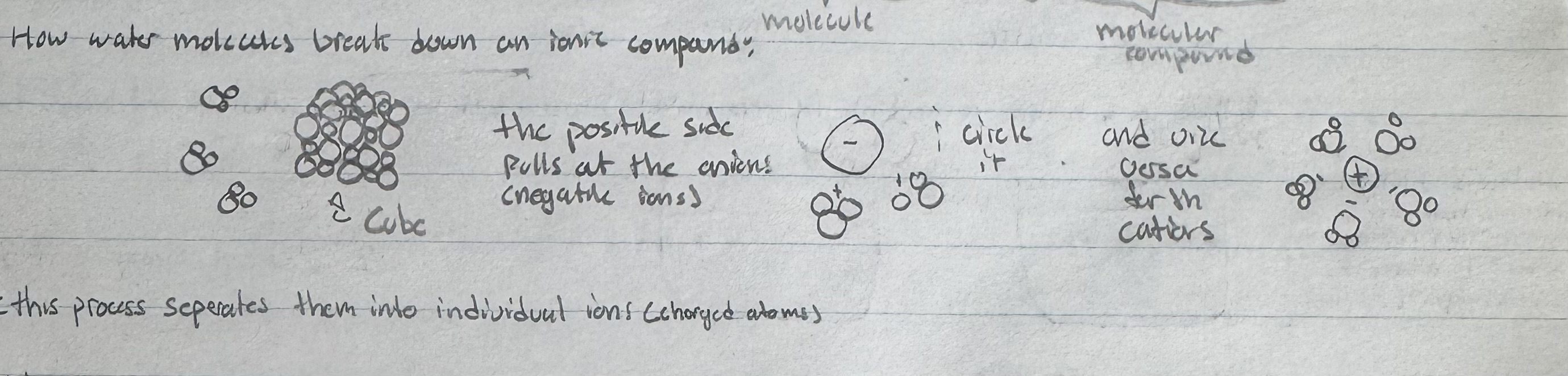
How do water molecules break down a molecular compound?
Since molecules (atoms held together by covalent bonds) are neutral, the H2O cannot break molecular compounds into individual atoms.
What they can do, is separate the molecules with uneven charges in the compound, due to unequal sharing of electrons molecule to molecule.
Why are dissolved ionic compounds conductive (electrolytes)?
The anion and cations are free moving, therefore can complete the circuit if positive and negative electrodes are placed within the solution.
The ions can carry the charge from one electrode to the other. Their movement creates the current.
Why are dissolved molecular compounds not conductive?
While molecular compounds can be broken up by targeting parts of uneven charge, the molecules will always have an equal number of electrons and protons, therefore neutral, and not able to carry current.
The particles generally stay in molecule form.
concentration
the amount of solute compared to the amount of the whole solution (including the solute).
One form of determining concentration is as follows:
10g salt in 90g water
concentration is (10/100) or 10% salt by mass
concentration (when naming solutions)
a solution with alot of solute; high ratio of solute to solution
dilute
solution with little solute; low ratio of solute to solution
Can a solute, and subsequently a solution, be unintentional?
Yes, PCBs or pollutants can be present in water
What are 5 properties that indicate relative concentration:
colour
taste
odour
colour intensity
transparency
What is one way to test concentration?
If solution is electrolyte, do a conductivity test→ more movement, higher charge(?). The rate of reaction can give a relative amount of concentration; more concentrated react more quickly.
qualitative properties
basic attributes you can observe with one or more of the 5-senses
What do chemical reactions involve between ions, atoms, and molecules?
collisions
collision reaction theory
if particles collide with a certain amount of energy, and a certain orientation, then the particles will be rearranged. The result is the chemical reaction.
When molecules collide, they may be broken into individuals atoms, and recombine in different arrangement forming new molecules.
Atoms move faster in liquids/gas than in solids.
Why do higher concentrations react more quickly and more strongly?
if there is higher concentration, there will be more possible collisions, and more possible chances for collisions
There are many ways to describe concentration, what are 3?
percent by volume (%v/v): ‘mL of solute / mL of solution * 100%’
volume of liquid solute dissolved in total volume of solution
parts per million (ppm): ‘g of solute/g of solution * 10^6 ppm’
used to describe levels of substance in very dilute aqueous solutions
molar concentration (molarity): ‘mol of solute(n)/L of solution(v)’ or ‘c=n/v’
amount of mols in total volume of solution
partitive division, how many mols spread out across x amount of total solution
what is the molar mass formula?
M=n/m
M= molar mass (atomic mass of the atoms)
n= mols of solute
m= mass in g
can be rearranged.
How would you rearrange the mol concentration formula to find mols?
c = n/v
n is “mols of solute”, isolate n
n= cv
Does dilution change the number of moles in a solution?
Dilution occurs when more solvent is added to a solution, this does not change the amount of solute. Although, it does change the concentration.
i.e solute = same, solvent = increase , change in solute/solution ratio
standard solution
solution of very accurately known concentration, that is diluted to make other solutions.
What is the dilution formula
c1v1=c2v2
C1= initial concentration
V1= initial volume
C2= final concentration
V2= final volume
to find dilution, you find the values that make this expression true through algebraic means
When to use dilution equation and molar concentration calculation?
Use the molar concentration formula when you’re starting with a solid solute.
Use the dilution equation when you’re starting with a solution and changing its concentration by adding solvent.
What does ‘aqueous’ mean?
The solution was formed with water as the solvent.
When to use each formula?
PPM: very small concentrations, identify mass of solute in (g), and volume of solution in (L)
Molar concentration: finding molarity, or number of moles in a solution.
Percent by volume: concentration of liquid-liquid solutions
Dilution equation: when changing concentration (not amount of solute) by adding/removing solvent
All can be rearranged to find missing variables.
What are some things to remember when balancing atomic equations
The coefficient will multiply by the subscript; 2O2 means 4 oxygen in total
For brackets, the coefficient will apply to each; 2Ag(NO2) means 2 silver, 2 nitrogen, and 4 oxygen
balancing is just ensuring both sides have the same amount of compounds
How to find subscripts from compound names?
Look at ‘hydrogen sulfide’ for example. We know that when forming bonds, sulfur takes on the charge of -2, or tends to gain two electrons to be stable.
Hydrogen takes on the charge on +1, or tends to lose one electron to be stable.
The path of least resistance for this compound garners 2 (+1) hydrogen for 1 (-2) sulfur. Therefore:
H2S (g)
What do coefficients represent?
Think of coefficients as moles, representing the ratio between amount of substances.
example:
in H2 + O2 → 2H2O
What’s the ratio ratio of O2 to H2O ?
O2 has the coefficient 1, H2O has the coefficient 2
1:2 mole ratio.
for every one O2 molecules, 2 moles of H2O are formed
Conversion factors
Because coefficients represent mole ratios, you want to find, say how many moles of Fe will react with 0.27 mols of O2 molecules:
mols given * coefficient of desired / coefficient of given = mols of desired
For example:
0.27 × 4/3 = 0.36
there is a 3:4 ratio between oxygen and Iron, for every 3 mols of oxygen, there will be 4 mols of iron. Since there is less than 3, you scale down.
Scaling down works by taking the given amount of a substance, determining what fraction it is of the original ratio, and applying that same fraction to the other substance to maintain proportionality.
Why is it important to look at the non-metal when deriving chemical formulas from names?
The non-metal gains electrons, so to streamline the process, you can determine how many electrons it needs from the metal.
How does ionic bonding work?
metal to non-metal bonds, or ionic bonding, is unique because of how metals are made up of positively charged particles within a ‘sea’ of floating electrons.
Occasionally some free floating electrons are lost to other atoms, which upsets the balance. The electrons are usually lost to atoms with a stronger affinity for them. The atoms receiving the electrons become negatively charged, and attract the positive ions from the metal.
oxidation
loss of electrons
example of a half reaction:
Ag(s) → Ag+(aq) + 1e-
reduction
gain of electrons
example of a half reaction:
Ag+(aq) + 1e- → Ag(s)
refinement; purifying metals from compounds
process of purifying metals from their compounds. Say in the case of Ag2S(aq), reduction is used to give the metal, Ag+, its electrons back, breaking the ionic bond. The neutral Ag compounds form metallic bonds thus creating pure silver.
spectator ion
the ion that does not undergo chemical change
Reduction agent
One that loses electrons
Oxidizing agent
One that gains electrons
What is the relationship between the most stable atom and the most reactive ion?
The most stable atom has the most reactive atom and vice versa.
Between an atom and ion, if the ion is above the atom, meaning it is more reactive to gaining electrons, while the atom is more reactive to losing electrons, there will be a spontaneous reaction.
Between an atom and ion, if the atom is above the ion, meaning it is more stable, less likely to oxidize, while the ion is also stable, less likely to reduce, there will be a non-spontaneous reaction.
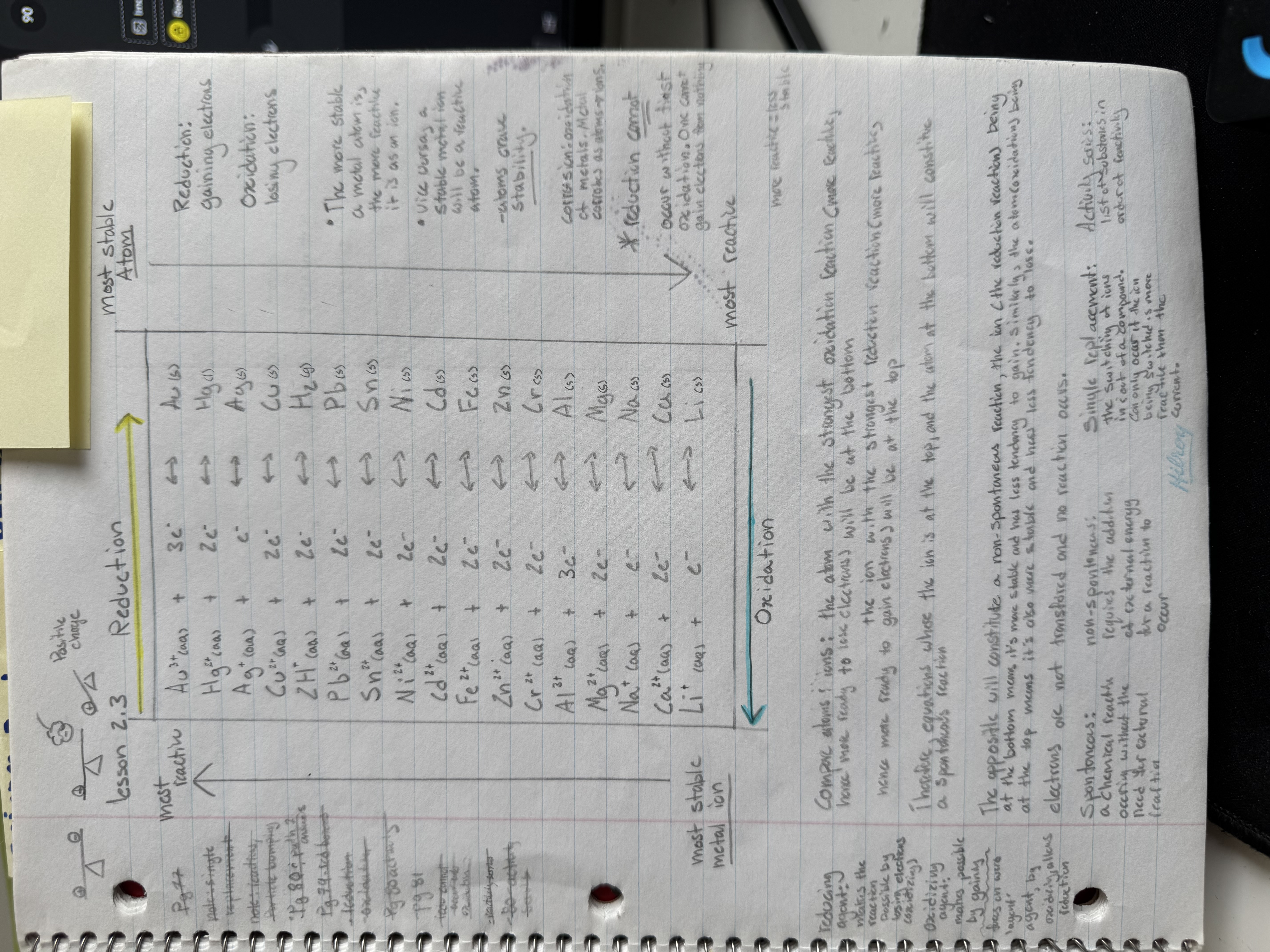
spontaneous
requiring no outside energy for a chemical reaction to occur
non-spontaneous
requires outside energy for a chemical reaction to occur.
single replacement
The switching of an ion in and out of a compound, and can only occur if the ion being switched is more reactive than the current ion.
Ag2S + N → Ag2N + s
*probably not accurate
galvanic/voltaic cells
devices that use a chemical reaction to create energy (reduction oxidation)
How does a galvanic/voltaic cell work?
Say copper and zinc are your electrodes. Cu ions are more reactive than Zn atoms are stable, therefore Zn has a weaker pull for electrons, enabling the stronger attracting force: Cu to take them.
We have a solution of ZnSo4 and CuSo4 in the respective electrodes containers to balance the equation with So4- ions. They also serve to provide a steady supply of Cu+ ions to start off the reaction by attracting Zn(s) electrons. The ZnSo4 provides a place for the Zn2+ ions to go after oxidizing (losing electrons).
the movement of Zn electrons constitutes an electrical charge.
Without a salt bridge, the Zn(s) atoms will keep losing electrons, becoming Zn+ ions, and detaching from Zn(s) to float in the ZnSo4 solution. The opposite will occur for Cu, as the Cu2+ ions will use zincs electrons to become neutral and bond with Cu(s), forming a bigger piece of copper.
Metallic bonding occurs between neutral metal atoms.
oxidation half reaction: Zn(s) → Zn2++ 2e-
reduction half reaction: Cu2+ + 2e- → Cu(s)
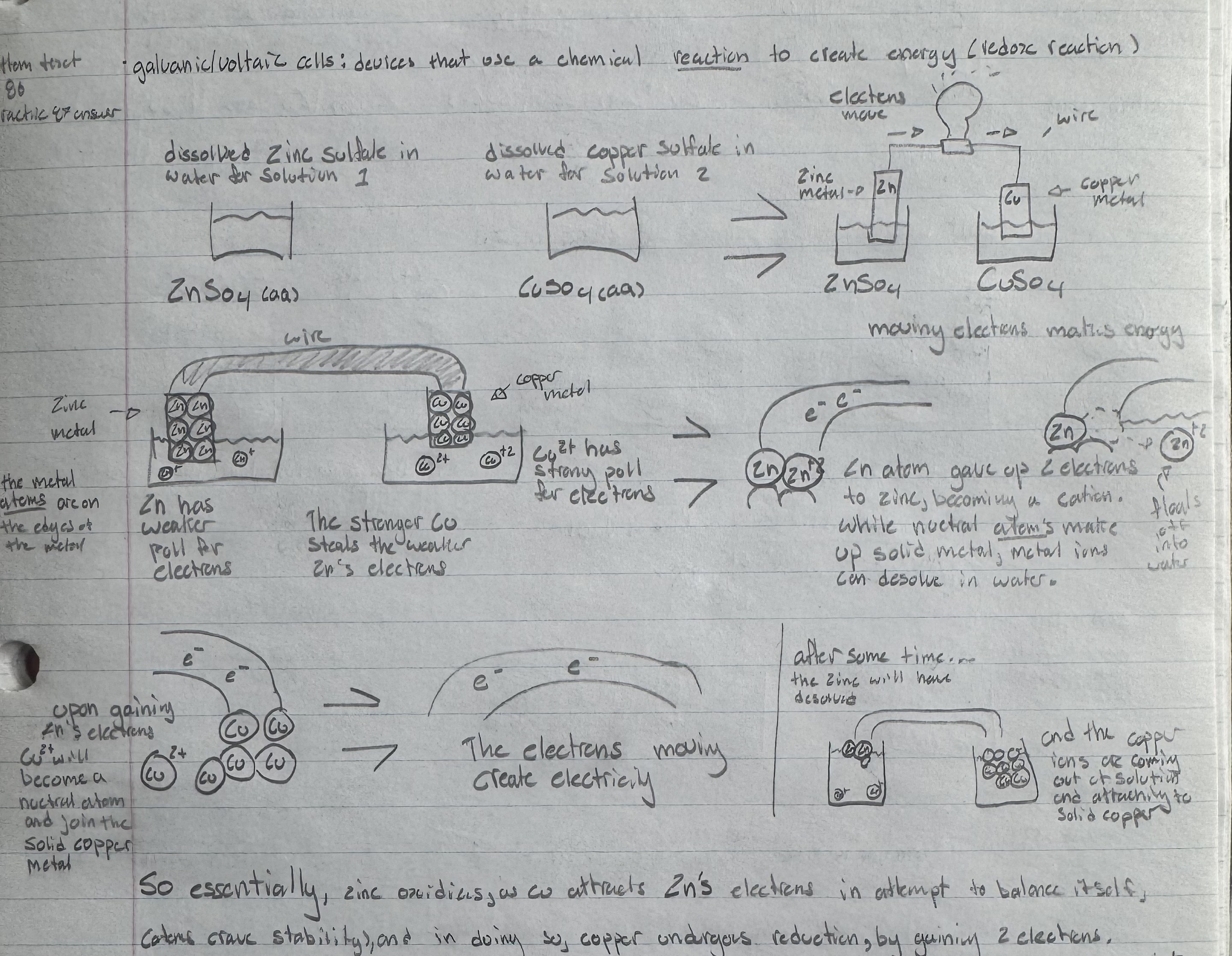
Cathode
The electrode which undergoes reduction.
Anode
The electrode which undergoes oxidation.