Section 2: Heterotrimeric G proteins
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Heterotrimeric G proteins
They are members of the superfamily of regulatory GTPases that are collectively known as G proteins
G proteins share a common structural motifs
They bond to guanine nucleotides GTP and GDP
Hydrolyze GTP → GDP + Pi
Why are heterotrimeric G proteins essential
Signal transduction
conveying a signal from exterior to interior of the call and / or cell to target
Vesicle trafficking
The growth of actin mircofilaments
Translation
protein targeting
as components of singal recognition particle (SRP) and the SRP receptor
Many heterotrimeric G proteins participate in signal transduction that consist of 3 major components
G protein-couples receptors (GPCRs)
Heterotrimeric G protein
Adenylated cyclase (AC)
G protein-coupled receptors (GPCRs)
They are transmembrane proteins that bind their corresponding ligand on their extracellular side and induces a conformational change on their cytoplasmic side
Where are heterotrimeric G proteins anchored to
Anchored to the cytoplasmic side of the plasma membrane
How are heterotrimeric G proteins activated
They are activated by a G-protein-coupled receptor (GPCR) when it binds to its corresponding ligand
This receptor recognizes / detects a signal from the exterior of the cell ams transfers via autophosphorylation to the interior of the cell and there is the G-protein
Where do heterotrimeric G-proteins convey its information to
Adenylate cyclase (AC)
Adenylate cyclase
It is bound to the transmembrane enzyme
It can be activated or inhibited by activated heterotrimeric G proteins
What does activated adenylate cyclase catalyze
It catalyzes the synthesis of adenosine-3’,5’-cyclic monophosphate (cAMP) from ATP
it facilitates the release of 2 phosphates
converts ATP → cAMP
ATP molecule
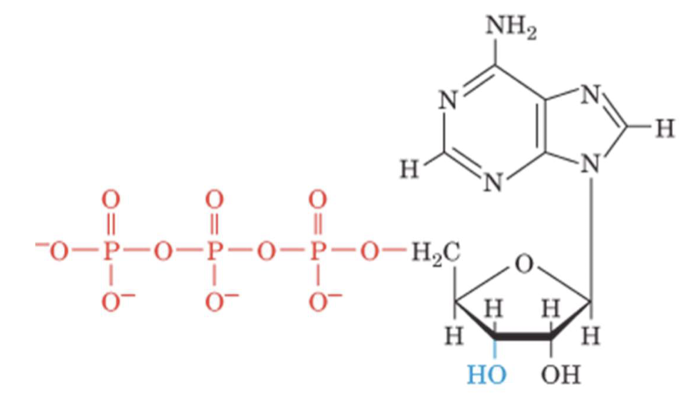
cAMP
cyclic adenosine monophosphate (cAMP)
it binds to a variety of proteins and activates numerous cellular processes
They act as secondary messengers
they are polar, freely
Adenylate cyclase active signaling system
Receptor protein recognizes external signal and is bound to the inactive heterotrimeric G-protein
∂-unit exchanges GDP w/ GTP and it is now active
∂ will dissassociate from ß and γ subunits and at the same time, it moves towards the adenylate cyclase
The activity conveyed by AC is the conversion of ATP → cAMP
Adenylate cyclase inhibitng signaling system
Inactive form binds to the receptor
GDP is replaced w/ GTP
∂-subunit has an inhibitory effect
How many isoforms of adenylated do mammals have
9
What are AC isoforms
tissues specific and have different regulatory properties
AC are transmembrane glycoprotein s
What are the conserved domains of Adenylated cyclase
Small N-terminal domain (N)
Transmembrane domane (M1)
2 consecutive cytoplasmic domains (C1a & C1b)
Transmembrane domain (M2)
2 consecutive cytoplasmic domans (C2a & C2b)
C1a & C2a
They form the catalytic core
They are 40% identical which allows them to associate w/ each other
C1a, C1b & C2a bind regulatory molecules
Cells can adjust their cAMP levels in response to a great variety of stimuli
depending on the concentration of cofactors, it allows the cell to regulate the amount of ATP we want to convert
What is the target for cAMP
Protein Kinase A (PKA)
Protein Kinase A (PKA)
It is made up of 4 subunits
2 regulatory subunits
2 catalytic subunits
PKA is inactive when all four are bound (PKA heterotetramer)
cAMP binds to the regulatory subunits
this causes dissociation of active catalytic monomers
What happens when cAMP binds to the regulatory subunit
It could result in the disassociation of the regulatory subunit from the catalytic subunit
The catalytic subunit then facilitates the phosphorylation of the target protein
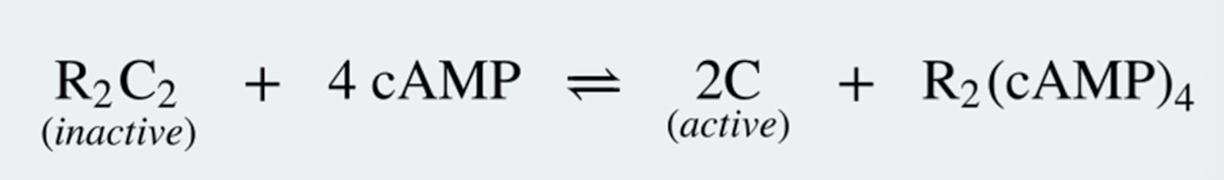
Protein Kinase A chemical formula
Intracellular concentration of cAMP
it determines the fraction of PKA in its active form
it is the rate at which it phosphorylates its substrates
Regulatory subunit of protein kinase A
it competitively inhibits its catalytic subunit
When the regulatory subunit is bound to the catalytic subunit, the entire protein is inactive
contains two homologous cAMP-binding domains (A & B)
also contains autoinhibitor segment
thsi resembles the catalytic subunit substrate
We can inhibit catalytic subunit by the absence of cAMP
once we have cAMP, they can start to bind w/ A & B domains and can no longer bind to catalytic subunit, therefore will be released
Inactive R2C2 complex
The authoinhibitor segments binds in the catalytic subunit’s active site
as does the inhibitory peptide → blocks substrate binding
Each R subunit cooperatively binds to
2 cAMP
What happens when the B domain lacks bound cAMP
It masks the A domain
It prevents A domain from binding cAMP
What happens when there is a binding of cAMP to the B domain
It triggers a massive conformational change
The A domain can bind cAMP
What happens if the A-domain is bound to cAMP
The release of the now-active C subites from the complex
How does cAMP activates the PKA
Activates by binding to the regulatory dimer
When it binds, the catalytic subunit C dissociates
This activates various cellular proteins by catalyzing their phosphorylation
Singaling is limited by the action of phosphatases and cAMP phpsphodiesterase
What limits the second messenger activity
Phosphatases
cAMP phosphodiesterase
are chemically based signaling system
the signal molecule must eventually be eliminated
in order to control the amplitude and duration of the signal
and to prevent interference with the reception of subsequent signal
cAMP-phosphodiesterases
hydrolyzes cAMP to AMP
PDE superfamily
included both cAMP-PDEs and cGMP-PDEs
they are encoded by at least 20 different genes groups into 12 families