DDT - All topics
1/158
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
159 Terms
What is a stroke
An instantaneous lack of blood in the brain which has resulted in a loss of neurological function. TIA (transient ischaemic attack) is known as a mini-stroke, and is typically a precursor to a stroke
Stroke risk factors
High blood pressure (normal 120/80mmHg), cigarette smoking (15% of all strokes in the UK), atrial fibrillation, diabetes, high cholesterol, carotid stenosis, obesity, Iatrogenic (illness caused by treatments or medical examinations), ethnicity, male risk higher than female
Arteries that bring blood to the brain
2 carotid arteries, vertebral and basilar arteries supply
Effects of stroke on regions of the brain
Anterior cerebral artery
Sensory/ motor control lower body
Mental state impairment
Middle cerebral artery
Upper limbs and face
Broca's Aphasia (LHS)
Posterior Cerebral artery
Visual field
Basilar artery
Locked in syndrome
Cerebellar artery (branched from basilar artery)
Poor co-ordination/ muscle tone
Dysphagia
What is infarction?
Tissue death is caused by a lack of oxygen due to a blockage in the blood supply. The most common area of infarction is the middle cerebral arteries
What is a penumbra and core
Penumbra is the area which could suffer damage from ischaemia BUT it is reversible. The core is damaged areas caused by infarction that cannot be repaired at all. Penumbra is also going to cause apoptosis, rather than core, which will cause necrosis
Inflammatory response to stroke
Neutrophils: Appear in the middle cerebral artery occlusion after a few hours
Lymphocytes: Appear within 24 hours (Makes tissue damage caused by stroke worse)
Microglia: Release pro-inflammatory cytokines
After a while, debris is digested via phagocytosis and beneficial cytokines may be released
Embolism meaning
Something that is able to block blood vessels
Pyknotic meaning
Nucleus shrinkage in necrotic cell
Angiogenesis meaning
Production of new blood vessels
Ways to diagnose strokes
FAST (Face dropped on one side, Arm(s) cannot be raised, Speech is slurred, Time to call 999) MRI, CT which need to be done as soon as possible to understand nature of stroke and deal with it
Treatment methods for strokes
Thrombolysis, thrombectomy, aspirin (stops platelet build up in blood vessels) as well as warfarin (antithrombotic treatment. Prevent the production of vitamin K, vital for thrombosis. Dangerous fo those with clotting factor defects however)
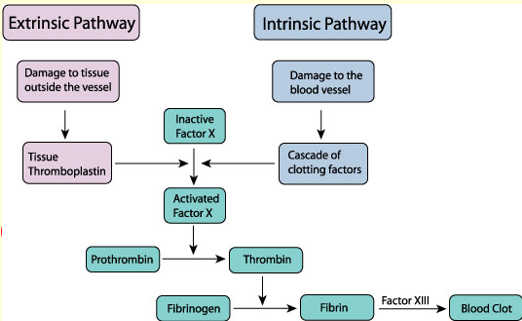
Overview of clotting (both intrinsic and extrinsic)
Window of opportunity for effective thrombolysis
4.5 hours from when the stroke first started
What is mechanical thrombectomy?
A catheter attached to a thin wire is put into the artery at the groin, which then goes to the brain, and is only used for those with severe ischaemic stroke, to remove a blood clot
What is haemorrhagic transformation
Where blood leaks into the brain, from a disrupted blood-brain barrier, which can worsen the effects of a stroke
What is anaemia?
Red blood cells are either: not being produced enough, not have a long enough lifespan (haemolysis), and increased blood cell loss. Can be it’s own disorder, and be caused by another disorder
Symptoms of anaemia
Lethargy, problems breathing, tachycardia (increased heart rate), and there can be no symptoms at all (asymptomatic)
How does the body compensate for anaemia?
Increased heart rate, increased production in immature red blood cells (reticulocytosis)
Causes of anaemia - Failure of productions (Haemoglobin) underlying conditions
Iron deficiency, haemoglobinopathy, anaemia of chronic disease (anaemia caused by an infection, kidney disease or cancer)
Failure of production - RBC underlying conditions
Bone marrow defects, renal failure, vitamin B12 deficiency
Increased RBC loss underlying conditions
Major blood loss could be a factor as well as diseases causing this like bowel disease/cancer where blood would be in faecal matter and stomach ulcers which can cause internal bleeding
Reduced RBC lifespan - structural defects
Sickle cell disease and spherocytosis (where RBC’s are spherical, not biconcave shape)
Reduced red cell lifespan - Caused by the immune system
Autoimmune haemolytic anaemia and incompatible blood transfusion
Reduced RBC lifespan - mechanical
Prosthetic heart valves (where blood leaks into the area between valve and heart tissue, after replacing heart values) hypersplenism (spleen is hyperactive, destroying too many RBC’s) and malaria (destroy’s RBC’s)
Reduced RBC life span - Enzyme defects
Pyruvate kinase and G6PD deficiency cause RBC’s to break down faster than usual
Types of blood cancers
Leukaemia, lymphoma, myeloma, and myelodysplastic syndrome
Ways to develop haematological diseases from the environment
Chemical exposures like benzene
Drugs like alkylating agents
Radiation which can damage cells and tissues, causing cancers
Infections like viruses (HTLV-1) and bacteria (Helicobacter pylori)
Ways to diagnose haematological diseases
Full blood count, haemoglobin test, blood film and flow cytometry
What is leukaemia?
Severe overproduction of WBCs, typically caused by bone marrow failures. Can be either chronic or acute
What is acute leukaemia and chronic leukaemia?
Acute leukaemia causes the aggressive proliferation of lymphocytes which invade organs and result in bone marrow failure. Chronic leukaemia is far more gradual, and affects mature cells
What is flow cytometry and how does it work
Flow cytometry is used to identify cells in homogeneous liquids, useful in cases like blood. Biomarkers are determined by using antibodies connected to fluorochromes for analysis. Also as light passes through the cell, it scatters, giving side and front scatter for additional information, like the size and complexity of the cell
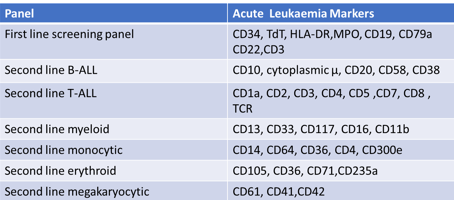
If a patient has biomarkers of CD34, TdT, HLA DR, CD10, CD19, and CD22 presents, which type of leukaemia would they have?
Second-line B-type acute lymphoid leukaemia (B-ALL)
Difference between Lymphoblastic and Myeloblastic
Lymphoblastic affects lymphocytes whilst myeloblastic affects granulocytes or monocytes
Treatment for CML, CLL, AML and ALL (Types of leukaemia)
Common cancer treatments like chemotherapy and stem cell therapy (to treat the abnormal bone marrow). Drugs which target the CNS are common, as leukaemia cells are normally always found in the CNS
What is MRD?
Minimal residual disease are small amounts of cancer left in the body after treatment, even if if seems that there is no disease left
Causes of abnormal haemostasis (coagulation)
Defects of any clotting factors, anticoagulation drugs, as well as low platelet counts (thrombocytopenia)
3 stages of haemostasis
The vascular phase (vasoconstriction to reduce blood loss), platelet phase (plugging the hole using platelets, using von Willebrand factor to bind platelets) and coagulation phase (stabilises plug with fibrin mesh as well as coagulation factors)
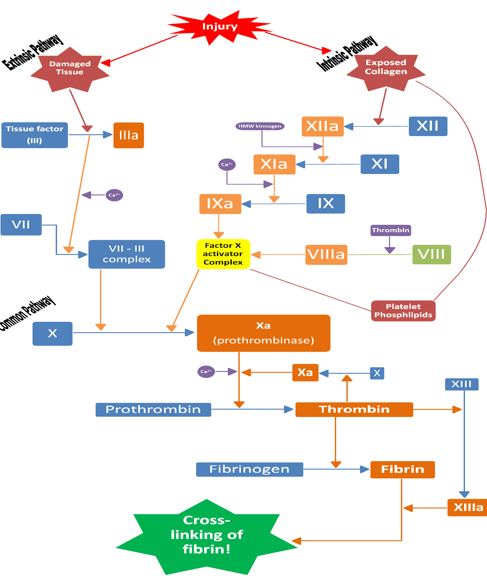
Fibrinogen requires what to convert to fibrin
Thrombin is required
Tests to detect coagulation abnormalities
Prothrombin time to test extrinsic clotting system effectiveness by adding tissue extract and calcium
Thrombin time for clotting after adding extra thrombin
Activated partial thromboplastin time (appt) where phospholipids, surface activators and calcium are added to test intrinsic pathway effectiveness
Fibrinogen to test the amount of fibrinogen in the body
Where are coagulation factors produced?
By the liver
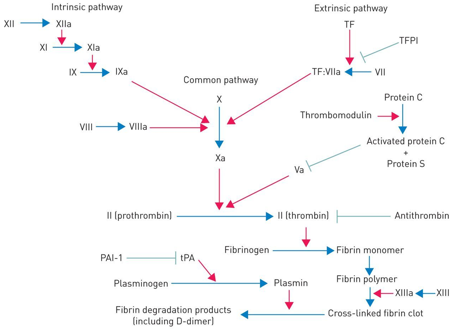
Coagulation factor inhibitors
Tissue factor pathway inhibitor (tfpi) which inhibits factors X, Vii and III tissue factor. Only released locally to prevent unnecessary clots
Antithrombin which inactivates thrombin, with the drug increasing it’s effects significantly
Protein C and S which inhibit cofactor V and VII
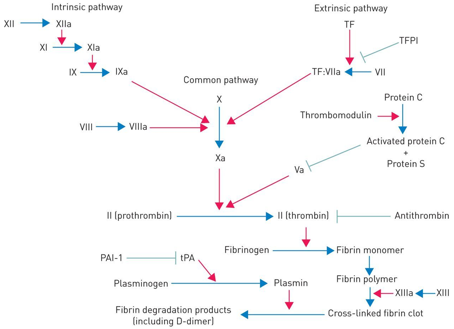
What is fibrinolysis, how does it work and how is it stopped?
Fibrinolysis is the breakdown of blood clots in the body, releasing plasmin, which inhibit fibrinogen, fibrin, factor V and VIII. Stopped by plasminogen activator inhibitor (PAI)
Treatment of abnormal haemostasis (too excessive)
If thrombosis is excessive, using anticoagulation drugs like warfarin or heparin, replacing fibrinogen via cryoprecipitation, and transfusion of platelets alongside fibrinogen side fresh plasma to replace the clotting factors. Also, treating underlying cause is the main objective
What is pharmacokinetics and 4 stages of pharmacokinetics?
How the body interacts with administered substances in the body. Absorption (how the drug enters the body), distribution (how the drug spreads through out the body), metabolism (how the drug is processed in the body) and excretion (how the drug is removed from the body) are the 4 stages.
Factors of absorption when administering drug?
Route of administration (Oral, intravenously, inhalation, sublingual, buccal), how the drug behaves and environment in the body like pH, blood flow.
4 mechanisms of absorption of drugs in GI
Passive diffusion, facilitated diffusion, active transport and endocytosis
What is bioavailability?
Rate and extent drugs reaches the systemic circulation system. Route of administration can greatly affect bioavailability
How are drugs filtered by kidneys (glomerular filtration)?
Drugs enter through renal artery to glomerular capillary plexus. Filtered by kidney, it goes through bowman’s capsule. Done by small drugs, not bound to albumin (proteins)
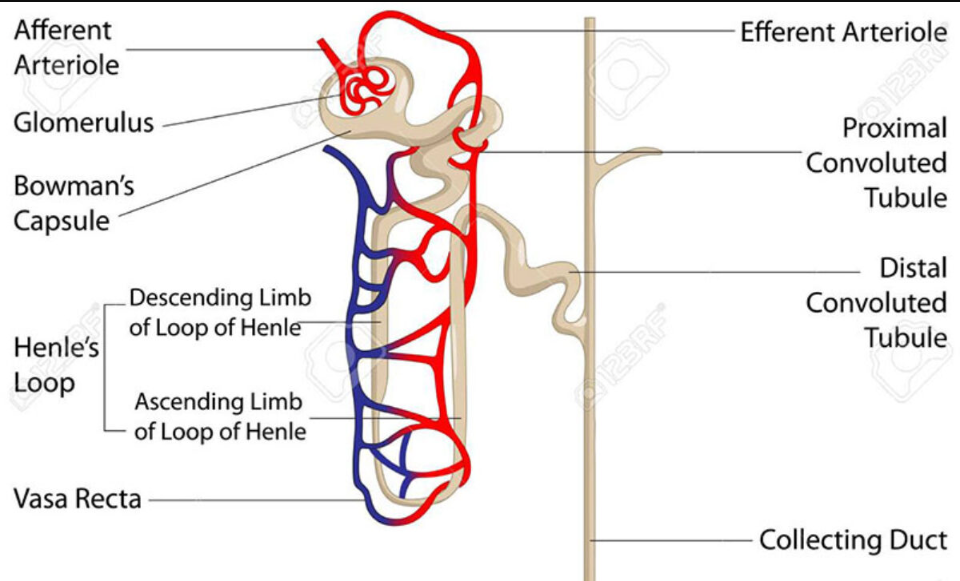
How are drugs filtered by kidneys (Active tubular excretion)?
If drugs have not been filtered, 2 active transport channels secrete anions and cations to get drugs to enter the proximal tubule. Competition between different drug can happen during this time
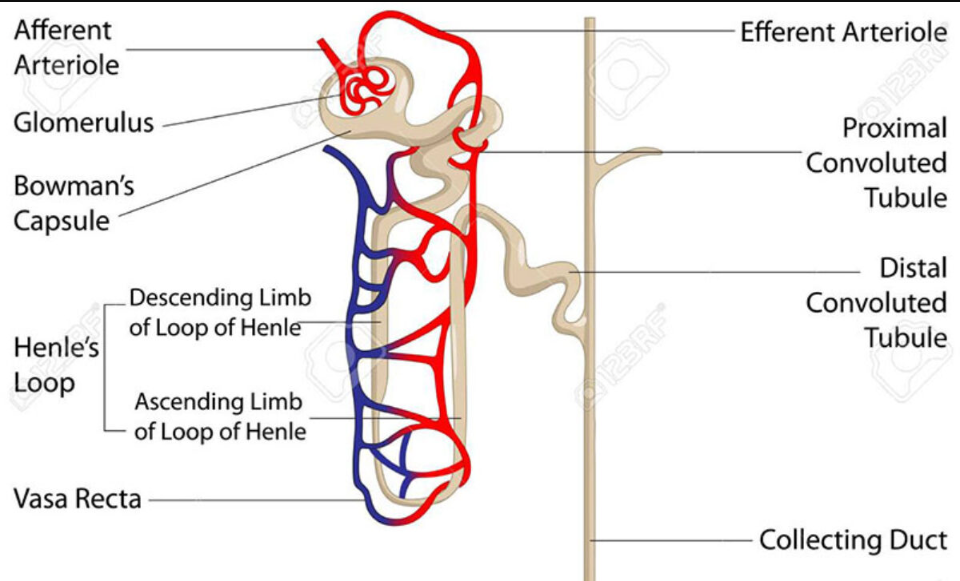
How are drugs filtered by kidneys (Distal tubular reabsorption)?
As drugs get to the proximal tubule, concentration of drugs increase, and some may be uncharged, so urine pH is manipulated to cause some drugs to be ionised, to prevent reabsorption, so it can be completely excreted
What is therapeutic drug monitoring
Measuring drug levels so it doesn’t become too toxic for the patient, during treatment
What are the 2 phases (and subphases) of xenobiotic metabolism in the liver
Phase I consists of hydroxylation (adding OH onto drug), reduction (electrons are added to drug to become more polar), oxidation (electrons are now removed?) and hydrolysis (breaks down drug using water into two component). Phase I essentially breaking the drugs into metabolising
Phase II consists of conjugation (where the drug is ionised), becoming easier to excrete
What is cytochrome p450 and Reactive Oxygen Species?
A monooxygenase (enzymes which add 1 oxygen into substrate), that adds oxygens to toxic molecules. Reactive oxygen species can be a by-product of the reaction, which are unstable, and are removed by enzymes and anti-oxidants.
What is pharmacodynamics?
How drugs act on the body and the effects they can cause, either by activating or blocking receptors or enzyms.
Different drug targets?
Ion channels (affects ion channel behaviour, either blocking them or opening them), G-protein coupled receptors (affecting enzyme activity and DNA transcription), enzymes (either blocking enzyme or give mimic substrate to enzyme, or create an active drug) and carriers/transporters (can block the transport of molecules or lead to fake compound)
Potency, efficacy, EC50, affinity and Kd definitions
Potency: Determines effectiveness of drug binding to receptor (higher potency = lower conc needed for effect to occur(
Efficacy: Limit before drug cannot cause any more effects, regardless of concentration given
EC50: Same as efficacy but limit is instead 50%
Affinity: How well drug can bind to receptor
Kd (dissociation constant): Where half the receptors have been binded and are being affected
Relationship between EC50 and Kd
If EC50 (50% effect) is higher than Kd (50% receptor bound to), there are spare receptors (Not every receptor needs to be bound to for the maximum effect to be reached)
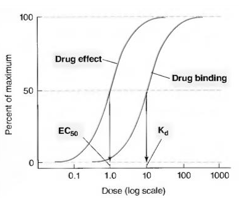
Relationship between affinity and Kd
Inversely proportional to affinity, so higher Kd = lower affinity, since less drug is required
Difference between agonists and antagonists?
Agonists bind to receptors, mimicking similar molecules, either by fully or partially activating the receptor. Antagonists just block the receptor, stopping a response from happening
Difference between full, partial and inverse agonist?
Full agonists produce maximum possible response when activating the receptor
Partial agonists produce a partial response when activating the receptor
Inverse agonists reduce the activity of the receptor
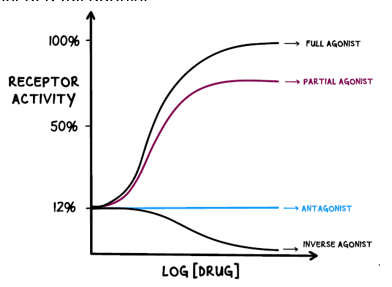
Difference between chemical, physiological and pharmacological antagonists
Chemical antagonists bind to another drug, rendering it useless, as it cannot bind to a receptor
Physiological antagonists produce a different effect to what another drug would have caused
Pharmacological antagonists bind to receptors, blocking them. It can either directly bind to the active site, or another site, which can also block an agonist
Types of pharmacological antagonists
Competitive: Binding to the same receptor against the agonist
Non-competitive: Binds to another site, causing permanent change to the agonist site, causing absolutely zero agonist effects
Ways to detect drugs
Liver function test: Testing ALP, AST, ALT, albumin and bilirubin levels to measure functions carried out by the liver
Mass spec: By ionising the samples, and separating the ions based on mass to charge ratio, the ions are detected which go through the data system to present the charge.
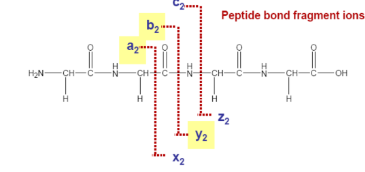
Where is the most common fragmentation likely to happen in this peptide bond?
At the b and y fragmentation area
If a compound is likely to degrade at high temperatures, would it be better to use GC/MS or LC/MS
LC/MS would be the better choice, as if the compound will degrade, accurate results cannot be obtained, like ketamine
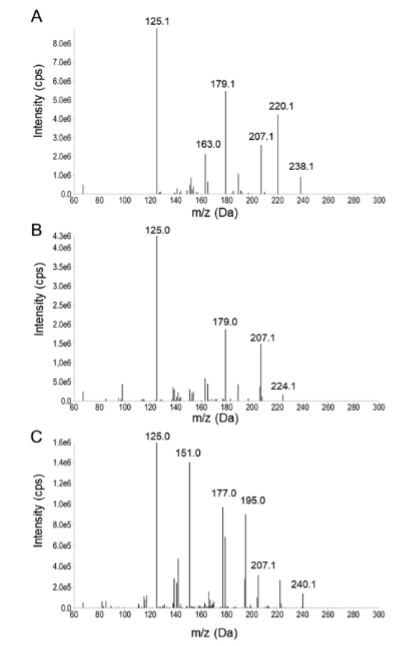
Ways to detect compound on mass spec graph
The last fragmentation typically shows the mass to charge ratio of the entire compound. Minusing 1 off this number will result in the molecular weight of the compound (238 would be ketamine, 224 would be norketamine and 240 cis-6-hydroxynorketamine, taking 1 off these resulting in the molecular weight for the compounds)
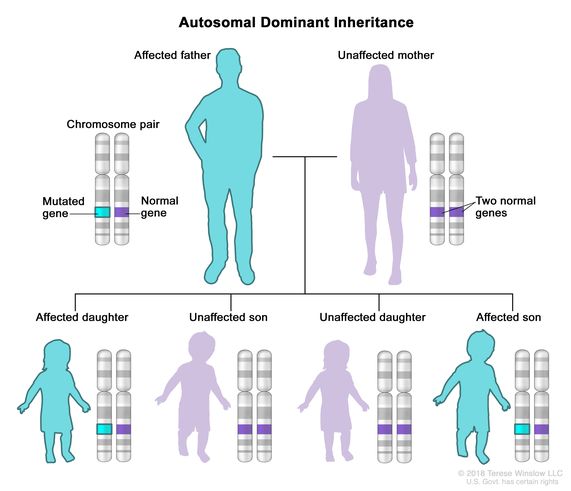
What is autosomal dominant
A pattern of inheritance where only one allele is required for the disorder / trait to be present.
What is autosomal recessive
A pattern of inheritance where two copies of a mutated gene, one from each parent, are required for the disorder to manifest in offspring. This means that the trait is expressed only when both alleles are present.
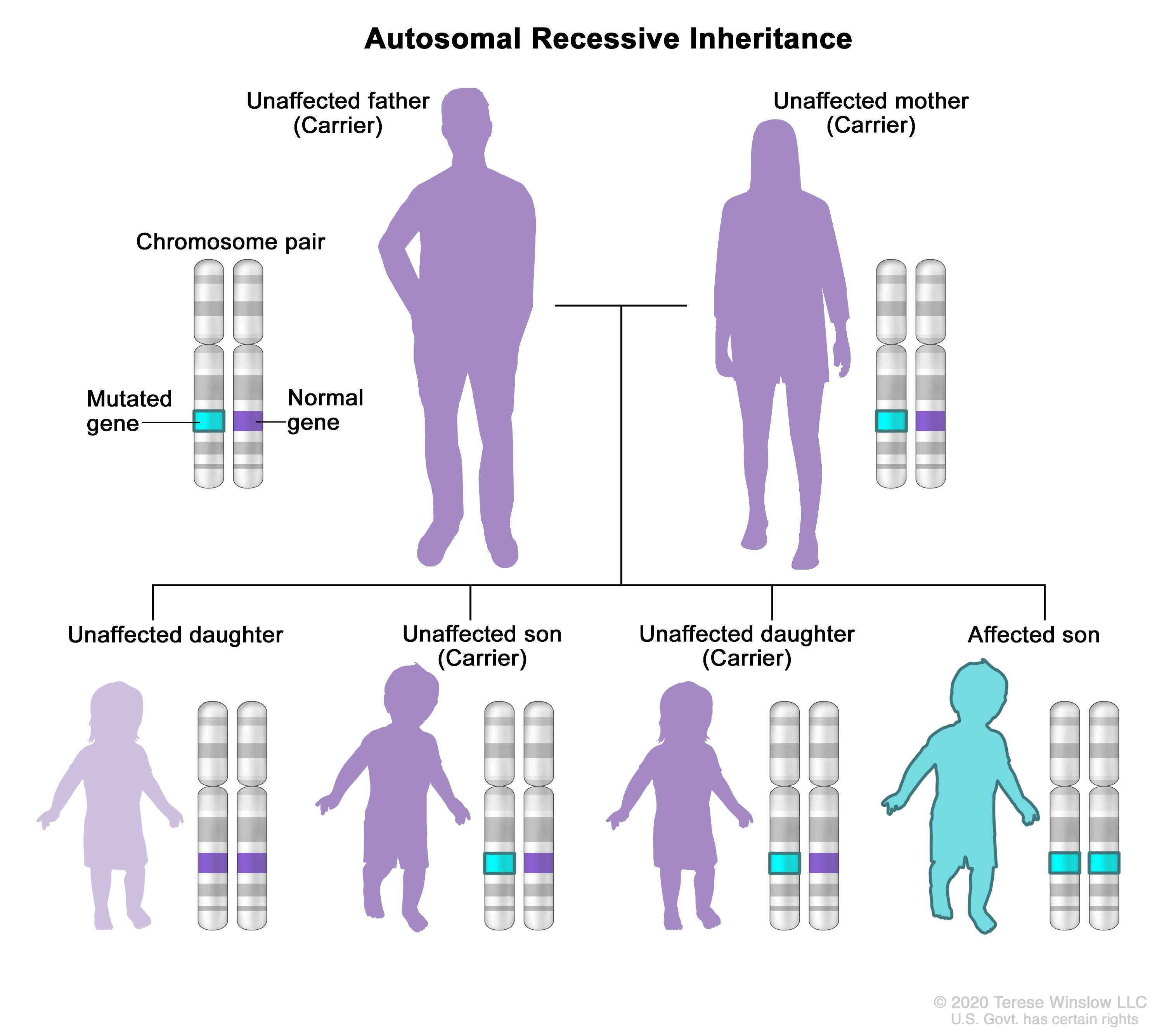
What is x-linked recessive
Genetic conditions associated with mutations in genes on the X chromosome. A male carrying such a mutation will be affected, because he carries only one X chromosome. A female carrying a mutation in one gene, with a normal gene on the other X chromosome, is generally unaffected.
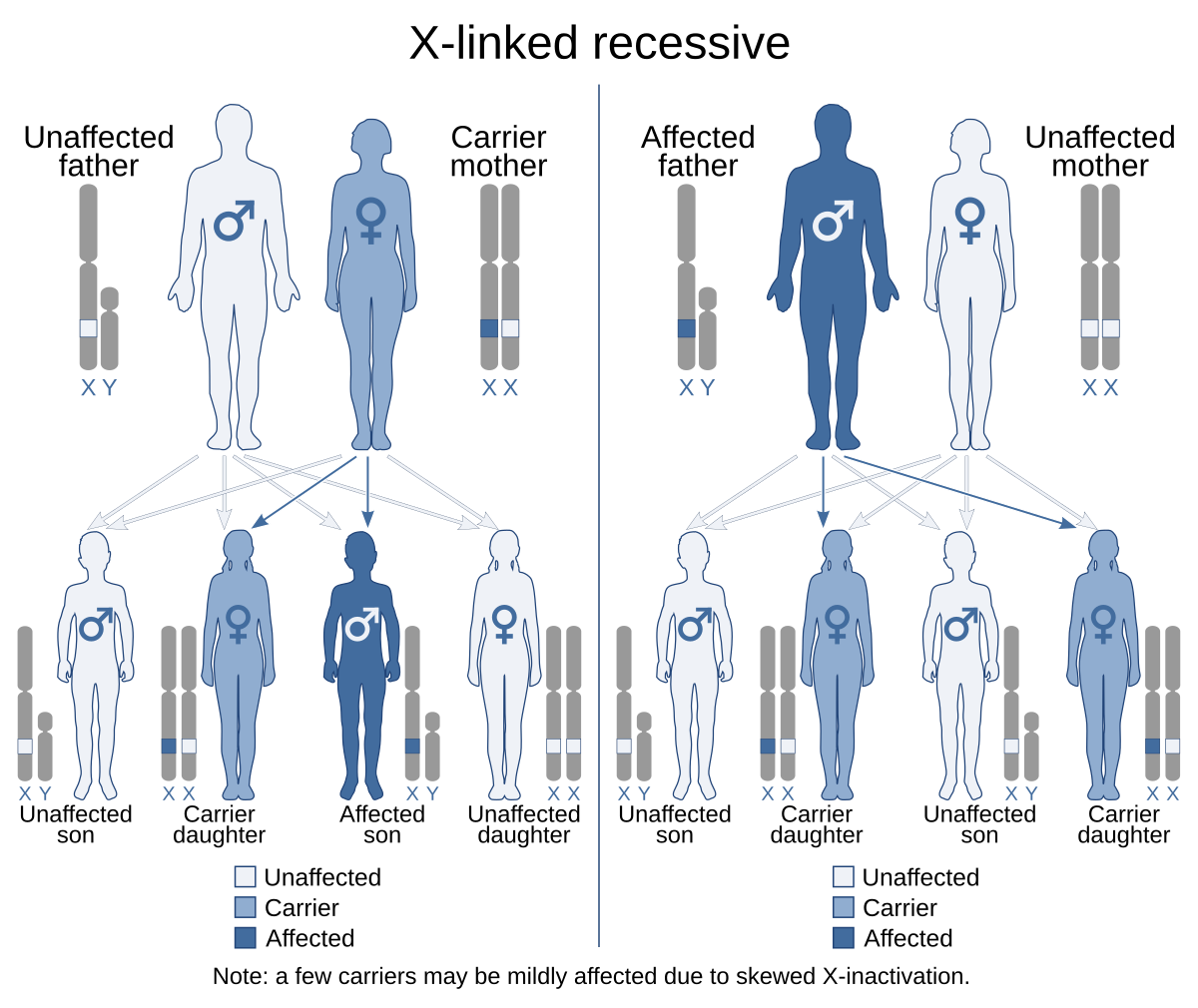
What is co-dominant in terms of genetics
A pattern of inheritance where both alleles in a heterozygote are fully expressed, resulting in offspring with a phenotype that is a combination of both traits. This occurs without one allele being dominant over the other.
What is germline mosaicism?
Germline mosaicism is a genetic condition where some of an individual's germ cells (sperm or eggs) carry a mutation, while others do not. This can lead to offspring inheriting genetic disorders even if the parent does not show symptoms.
What is somatic mosaicism?
Somatic mosaicism is a condition where an individual has two or more genetically different cell lines within their body, resulting from mutations that occur after fertilization.
What is a nonsense mutation?
Where a stop codon is prematurely present due to amino acid change
What is a missense mutation?
Replacement of one amino acid to another in a protein
What is a splicing mutation?
Where exon to intron sequences are changed, leading to either partial or complete loss of exons, leading to shortening of proteins
What is a frameshift mutation?
A genetic mutation caused by the insertion or deletion of nucleotides in a DNA sequence, altering the reading frame of the genetic code. Also splicing mutations can cause this to occur as well.
Changes in the promoters will lead to?
Change to a protein sequence?
No change to protein sequence
Change in splicing region
Change in protein expression
No change in the gene at all
Change to a protein sequence?
No change to protein sequence
Change in splicing region
Change in protein expression
No change in the gene at all
Loss of control during transcription or will need to use an alternative promoter during transcription
If a single base pair change occurs in an exon it will cause?
Change to protein sequence
No change to protein sequence
Change in splicing region
Change in protein expression
No change in the gene at all
Change to protein sequence
No change to protein sequence
Change in splicing region
Change in protein expression
No change in the gene at all
This occurs as it will either change or remove exons, insert or remove stop codons and alter splicing sites as well as affecting the open reading frame.
If a single base pair change occurs in an intron it will cause?
Change to protein sequence
No change to protein sequence
Change in splicing region
Change in protein expression
No change in the gene at all
Change to protein sequence
No change to protein sequence
Change in splicing region
Change in protein expression
No change in the gene at all
This will occur as it will alter the splicing site
What is gain of function?
Changes in gene function, affecting protein production, may become active and enhanced to work in other ways. It is dominant when it comes to inheritance
What is loss of function?
Loss of function is where gene function is either reduced, or completely inactive, even if proteins are produced. This is typically recessive in terms of inheritance
What is an inborn error of metabolism
A rare genetic disorder where the body is unable to break down food, due to a lack of enzymes /
A heterogeneous disorder where metabolic pathways are inactive, and carbohydrates, fatty acids and proteins are either not stored or broken down effectively
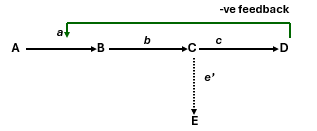
If enzyme c was missing what would occur to the other parts in the pathway?
There would be no production of D as there is no enzyme to convert C to D. There would be a build up C and E as it cannot be converted to anything else, and A and B would possibly build up as well if the path is reversible. Also there would be no negative feedback loop either
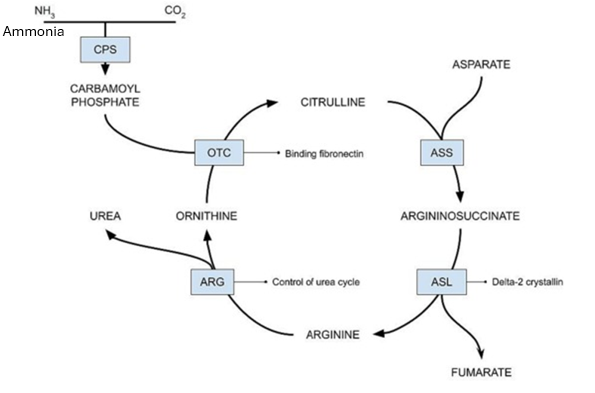
If the OTC enzyme has a loss of function mutation, what would build up as a result of this?
Carbamoyl phosphate would build up as it would not be converted into citrulline
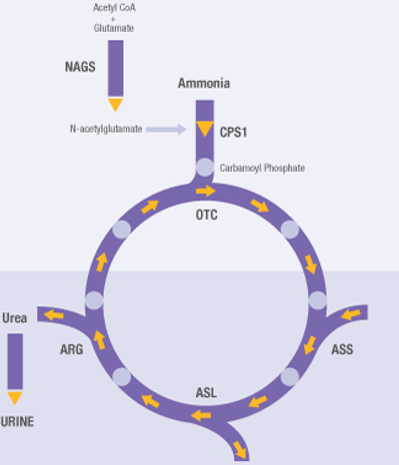
Which loss of function mutation would have a greater malignancy, and cause more damage to the body:
NAGS (Converts ammonia, which is incredibly toxic) or ASS (Converts citrulline, which is not found to be toxic)
NAGS would be far more dangerous, as it will cause a build up of ammonia, leading to hyperammonaemia, leading to coma and possible death. The ASS enzyme is still important, but a LOF is far less impactful than NAGS.
2 patients are showing mutations linked to the CPS1 enzyme, vital for the urea cycle. One patient has 1 nonsense and 1 missense mutation, while another patient has 2 nonsense mutations. Which patient has the more severe symptoms?
The 2 nonsense mutations are far more severe than the other patient, as no enzyme is produced at all. This leads to a build up of ammonia, and can lead to death. The 1 missense and 1 nonsense patient is still going to produce enzymes, but will have less activity than a useless person, but it will not result a risk to life. This would have a similar result for any pathway in the body.
What is the Guthrie test (neonatal screening), amniocentesis and also chorionic villus biopsy and how it’s performed.
Guthrie test is a prick to the baby’s heel post birth to collect blood, to test for 9 serious diseases. Developed in 1959 and mid 60’s was used common for newborn screening
Amniocentesis is the collection of amniotic fluids post week 15, since it is filled with foetal keratinocytes (Skin cells)
Chorioni villus biopsy is only used if it is to be believed if there is a possible miscarriage, or cause serious harm to the baby, like abnormal chromosomes and chromosome counts. Takes tissue from the placenta
Methods of detecting genetic diseases
Ideograms / Karyotype that show images of chromosomes, to detect mutations
FISH (fluorescent in situ hybridisation) that uses fluorescent labelled DNA to detect mutations as well, using stains and counterstains to highlight different locus or chromosomes
PCR and sanger test make up the entire genome
ARMS based PCR detects specific mutations you’re looking for quickly
Next gen massively parallel sequencing screen DNA using PCR and making daughter strand extend by a nucleotide at a time
Small scale, fast results would require which type of genetic testing method?
Next generation would be the fastest method to use, as it can give results in seconds. However, if time is not a constraint, either FISH or Karyotype would be the next best option
When would PCR and sanger testing be the best choice?
When time is not an issue, and when you want incredibly in depth answers, to detect abnormalities that no other test can detect (The gold standard of genetic sequencing)
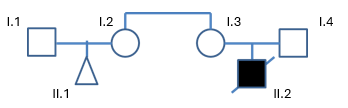
Using the image below who you need to test if the genetic disorder for child II.2 (Male) has a genetic disease that fits either of these diseases:
X-linked recessive
Autosomal Recessive
You would need to test the I.3 for X-linked recessive as they are carriers, and cannot be affected by the disease. Male to male transmission is also not possible with this, so you would not need to test the male.
For autosomal recessive, both parents would be tested (I.3 and I.4), since 2 recessive alleles are needed
What is gene therapy?
Giving someone medical care by altering / modifying their genes
Give a few examples of gene therapy
Haematopoietic stem cell gene therapy - Can treat immunodeficiency diseases
Immunotherapy for cancer - Modifying patient T cells to attack cancer cells
The liver - Can help it to not be affected by viruses, as well as help to treat other diseases
These are just a few examples, there are much more to pick from
What are some treatments for those who have inborn errors of metabolism, like phenylketonuria?
With phenylketonuria, there must be strict phenylalanine intake whilst adolescent and brain still developing (Maybe even further beyond)
Giving patient tyrosine as supplement which is highly deficient in those affected by phenylalanine
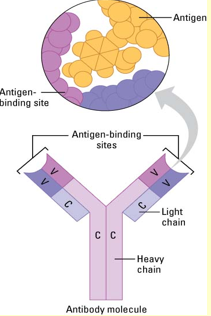
Structure of an antibody
Made from 4 polypeptides forming a Y shape, with the V region being able to alter any shape that fits the specific shape of the antigen. The C region will never change, and is always constant
Difference between monoclonal and polyclonal antibodies
Monoclonal only have one epitope (binding site of antigen) whilst polyclonal antibodies have multiple epitope for one antigen
Preparing polyclonal and monoclonal differences
Polyclonal preparation is generally easier, and cheaper but will yield a variety of antibodies, but monoclonal results in better antibodies but is more expensive and difficult
Different types of diagnostic based antibody technique
Immunohistochemistry would be used for tissue samples, ELISA for liquid samples and immunoprecipitation for complex mixture samples
Types of labelling antibodies
Using enzymes, fluorescent and radioisotopes are ways to label antibodies during immunoassays