Yr 9 Chem test - Psychology, flame test, Properties of Ionic Compounds, Properties of Metals
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
What is the atomic number of an element, and where is it found in an atom?
The atomic number is the number of positively charged protons in the nucleus of an atom.
How do you find the number of protons of an element from their atomic number?
The number of protons in an atom is equal to the atomic number.
How do you identify the number of electrons from the atomic number?
In a neutral atom, the number of electrons equals the number of protons. And the number of protons is the atomic number.
Explain how the atomic number of an element determines its position in the periodic table.
The atomic number is the number of protons in an atom.The periodic table is arranged in order of increasing atomic number, so each element's position is based on how many protons it has.
What is the mass number of an atom, and what does it represent?
The mass number is the total number of protons and neutrons in the nucleus of an atom.It represents the total mass of the atom's nucleus.
How do you calculate the amount of neutrons in an atom from the mass and atomic number?
Number of neutrons = Mass number − Atomic number
Write the atomic symbol for an element between 1 and 20
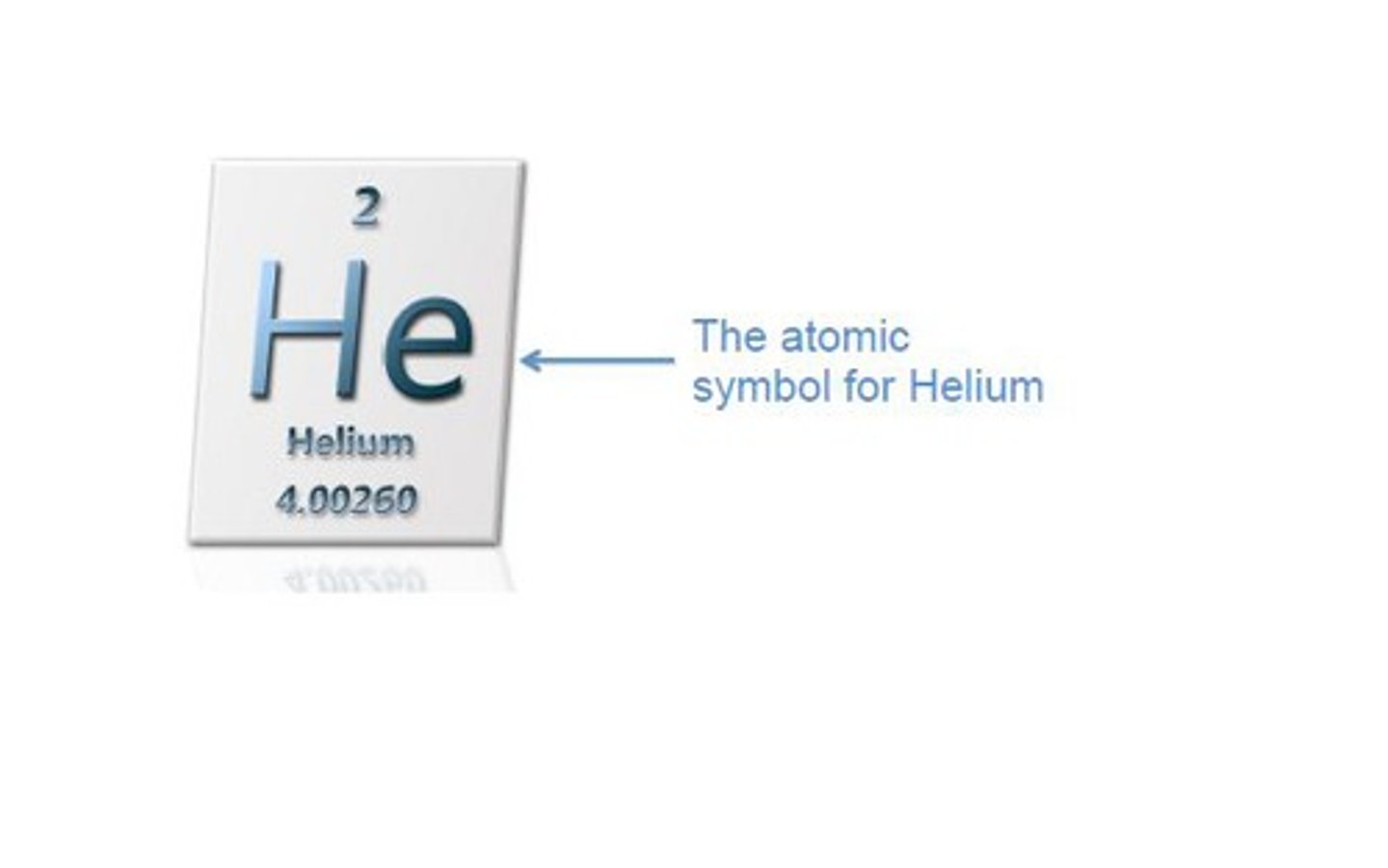
Write the istope atomic symbol for elements between 1 and 20
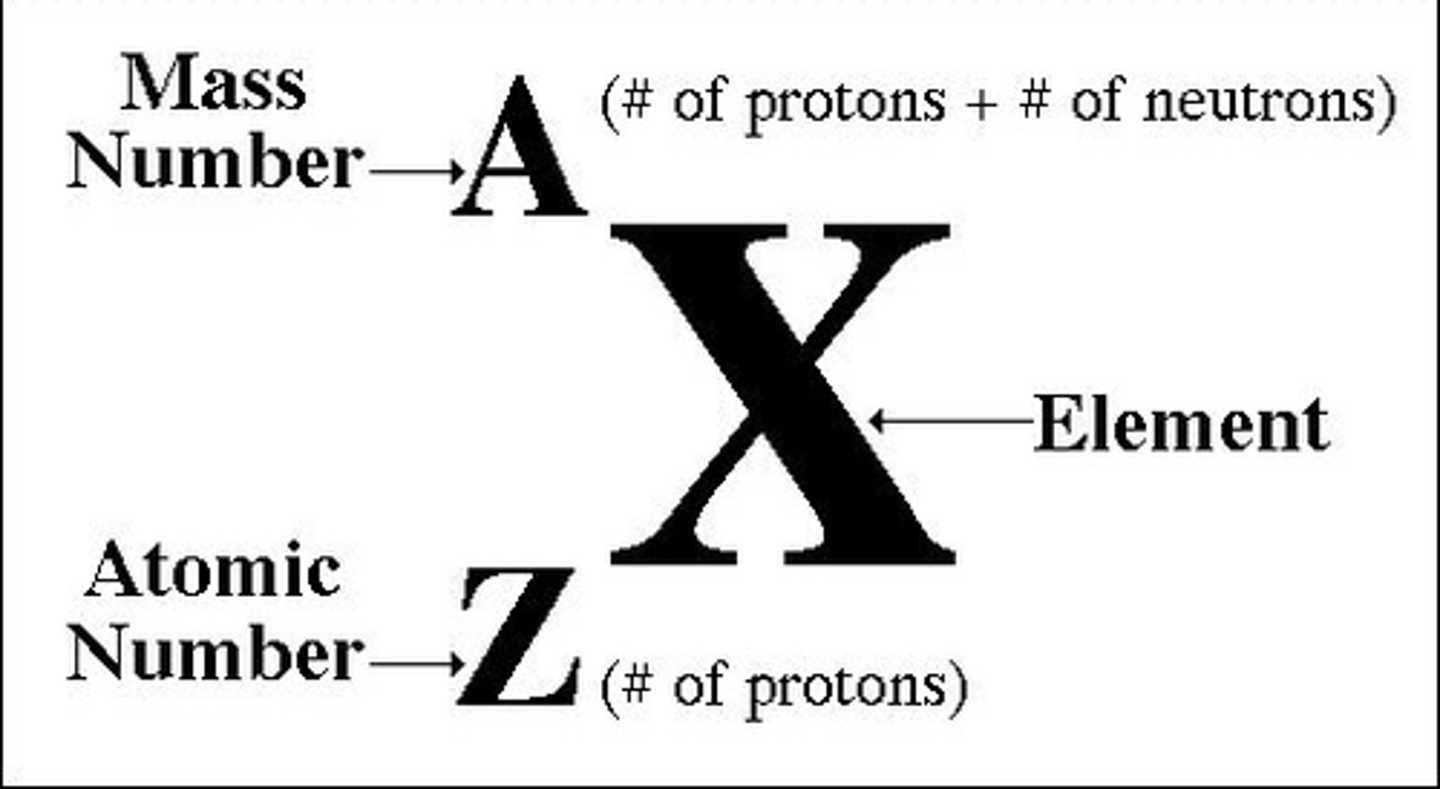
What is an isotope
Isotopes are atoms of the same element that have the same number of protons (same atomic number) but different numbers of neutrons.
Define an electron, including its charge and relative mass.
An electron is a negatively charged particle with a charge of -1. It has a negligible mass compared to protons and neutrons.
Draws bhors electron configuration for a neutral atom up to 20
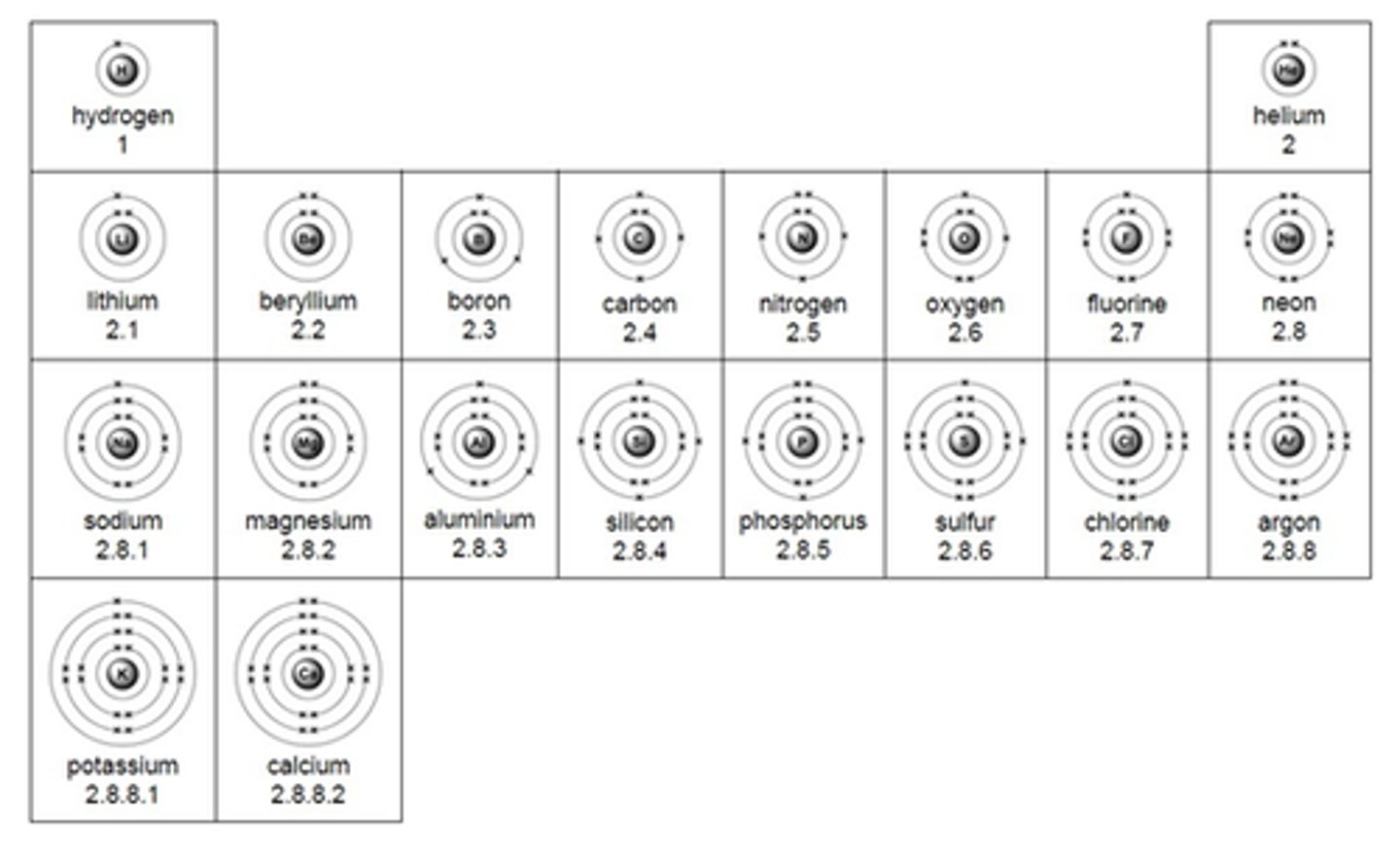
Write bhors electron configuration for a neutral atom up to 20
eg. calcium: 2,8,8,2
Explain how an element's electron configuration determines its position in the periodic table.
The electron configuration shows how electrons are arranged in an atom's shells and subshells.
- The period number corresponds to the number of electron shells occupied.
- The group number (for main group elements) corresponds to the number of electrons in the outer shell (valence electrons).
Why are transition metals placed in the center of the periodic table, and what distinguishes their electron configuration from other elements?
- Unlike main group elements that fill s or p subshells, transition metals have incomplete d subshells.
- Their placement reflects these distinct electron arrangements.
Write the chemical formulas for the following polyatomic ions:
Hydroxide
Nitrate
Sulfate
Ammonium
Model Answer:
Hydroxide: OH⁻
Nitrate: NO₃⁻
Sulfate: SO₄²⁻
Ammonium: NH₄⁺
Determine the valency of elements in the following groups based on their position in the periodic table:
Group 1
Group 2
Group 16
Group 17
1. Group 1 elements have 1 valence electron, so their valency is 1.
2. Group 2 elements have 2 valence electrons, so their valency is 2.
3. Group 16 elements have 6 valence electrons, so their valency is 2 (they tend to gain 2 electrons to complete their outer shell).
4. Group 17 elements have 7 valence electrons, so their valency is 1 (they tend to gain 1 electron to complete their outer shell).
Draw electron transfer diagrams showing the transfer of valence electrons between:
A Group 1 metal and a Group 17 non-metal (e.g., Sodium and Chlorine)
A Group 2 metal and a Group 16 non-metal (e.g., Magnesium and Oxygen)
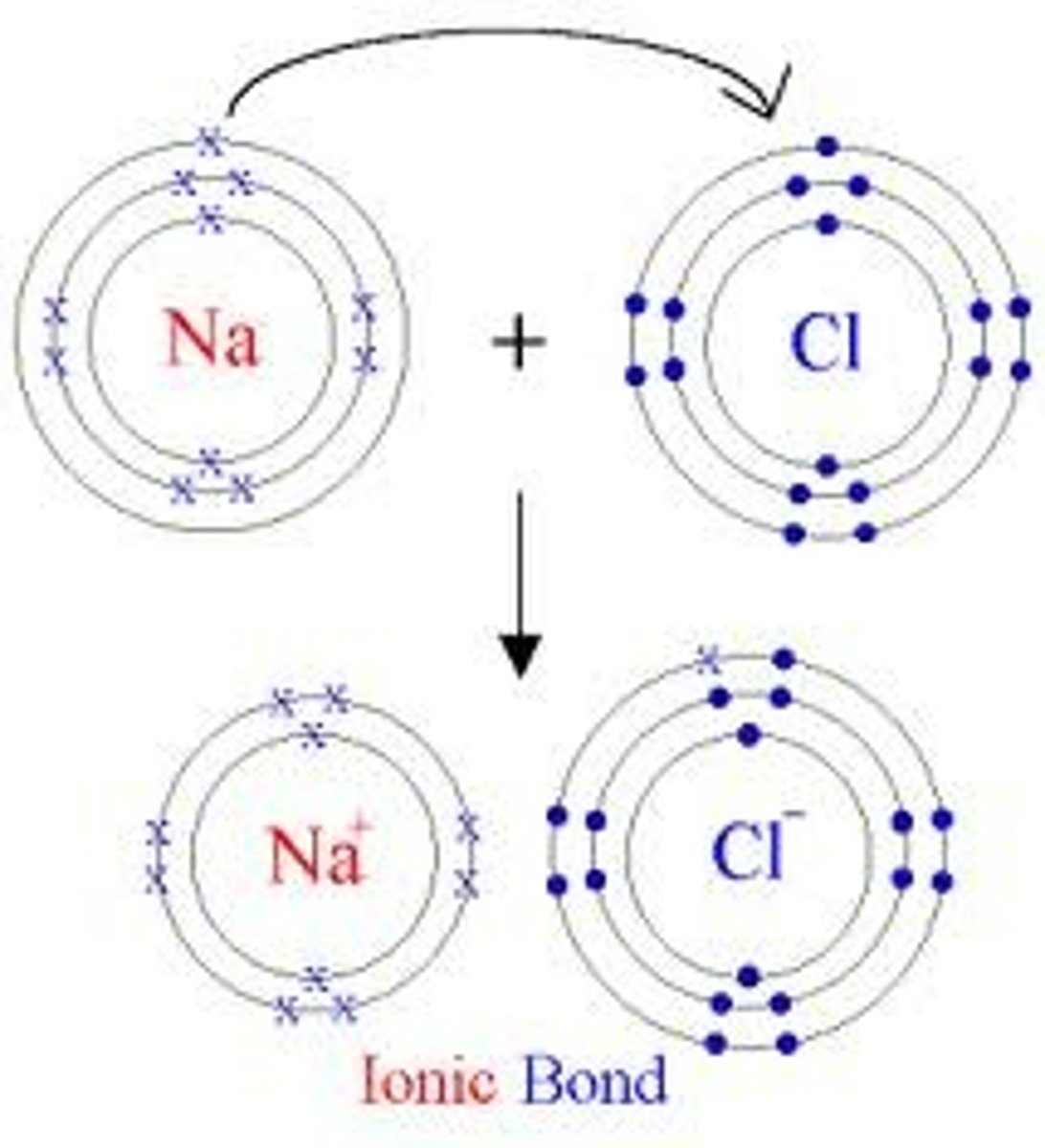
Explain why a metal forms a positive ion while a non-metal forms a negative ion, using the concepts of ionisation energy and electronegativity.
Metals have a low ionisation energy, so it easily loses one electron to achieve a stable electron configuration, making it positive.
Non-metals have a high electronegativity, so it tends to gain an electron to complete its outer shell, making it negative.
Define the terms cation and anion.
A cation is a positively charged ion formed when an atom loses electrons.
An anion is a negatively charged ion formed when an atom gains electrons.
Write an ionic compound formula containing polyatomic ions
eg. Magnesium and Chloride: MgCl₂ (because 1 Mg²⁺ balances with 2 Cl⁻)
Aluminum and Oxide: Al₂O₃ (because 2 Al³⁺ balance with 3 O²⁻)
Why do ionic compounds have their formulas written in the simplest whole-number ratio rather than showing the exact number of ions in the solid?
The formula shows the simplest ratio of cations to anions that balances the charges, representing the proportions needed to maintain a neutral, stable lattice.
What is ionic bonding and between which types of elements does it occur?
Ionic bonding is the force of attraction between positively charged metal ions and negatively charged non-metal ions. It occurs when a metal transfers electrons to a non-metal, resulting in the formation of oppositely charged ions that are held together by electrostatic forces.
Draw a particle model of simple ionic lattices in solid form
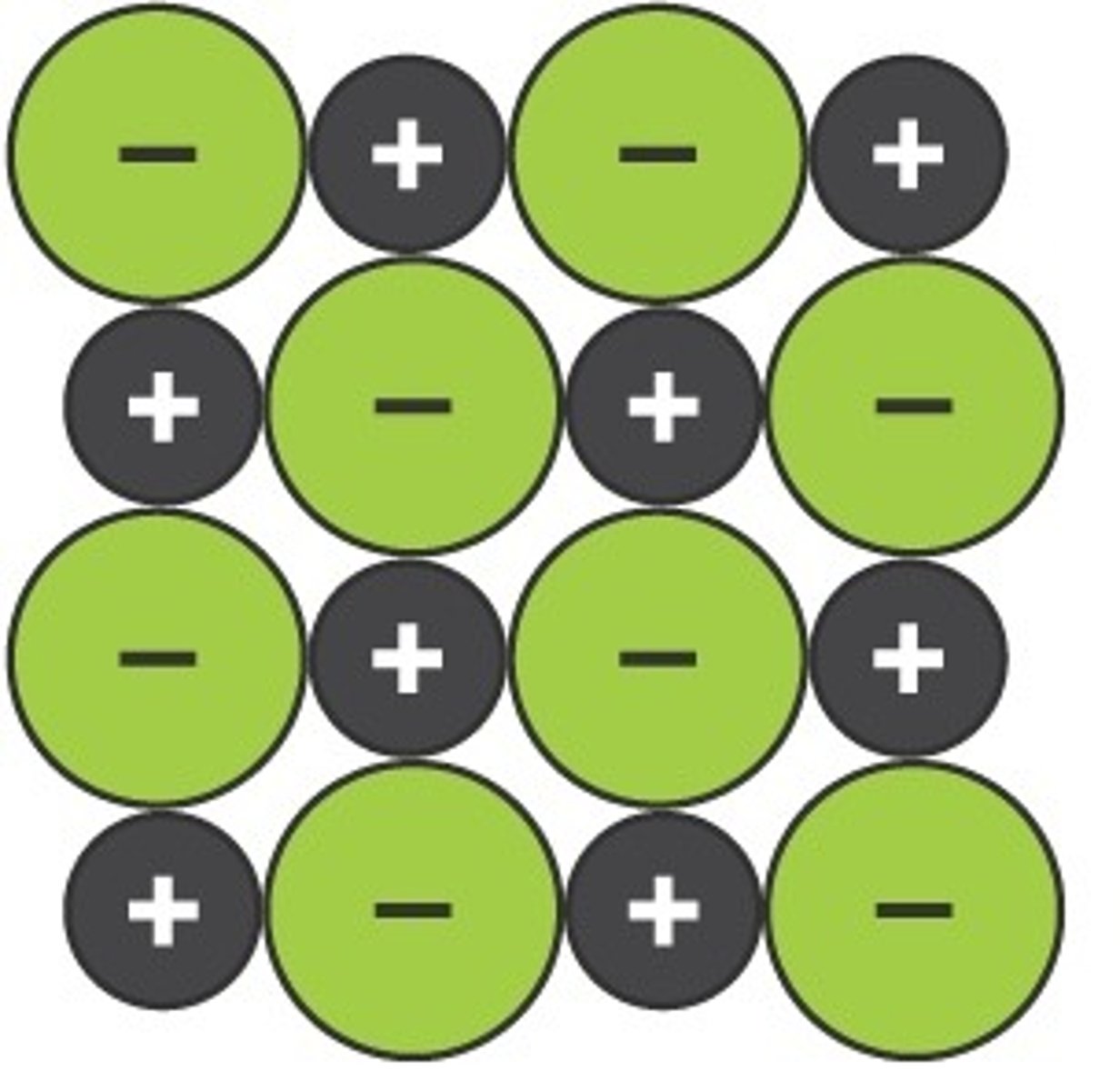
Explain why ionic compounds are hard and brittle, have high melting points, and conduct electricity only when molten or dissolved.
Hard and high melting point: Ionic compounds have strong electrostatic forces holding ions together..
Brittle: They are brittle because shifting layers causes like-charged ions to repel and break the lattice.
Conductivity in solid: In solid form, ions are fixed(can't move freely) and cannot conduct electricity.
Conductive in liquid/molten form: melted or dissolved, ions move freely and conduct electricity.
Strengths of the ionic bonding model
1. Explains high melting and boiling points:The model shows strong electrostatic forces between ions, explaining why ionic compounds need lots of energy to melt or boil.
2. Accounts for electrical conductivity:It predicts that ionic solids don't conduct electricity, but molten or dissolved ionic compounds do, because ions become mobile.
3. Describes crystal lattice structure:The model explains the regular, repeating 3D arrangement of ions that gives ionic solids their hardness and brittleness.
4. Helps predict formulas and charge balance:It clarifies how metals lose electrons and non-metals gain electrons, allowing prediction of chemical formulas.
Limitations of the Ionic bonding model
1.Simplifies ion shapes and interactions:The model often shows ions as perfect spheres and ignores complexities like polarization and partial covalent character.
2.Ignores electron cloud behavior:It doesn't show how electrons are actually shared or transferred on a quantum level.
3. Limited for complex or covalent-like compounds:Some compounds have bonding with mixed ionic and covalent character, which this model doesn't fully explain.
4.Doesn't account for variations in lattice energy:Strength of ionic bonds can vary with ion size and charge, but the model doesn't show this clearly.
Define metallic bonding and state between which types of atoms it occurs.
Metallic bonding is the type of chemical bonding that occurs between metal atoms, where positively charged metal ions are surrounded by a "sea" of delocalized electrons.
Draw a particle model of simple metallic lattices in solid form
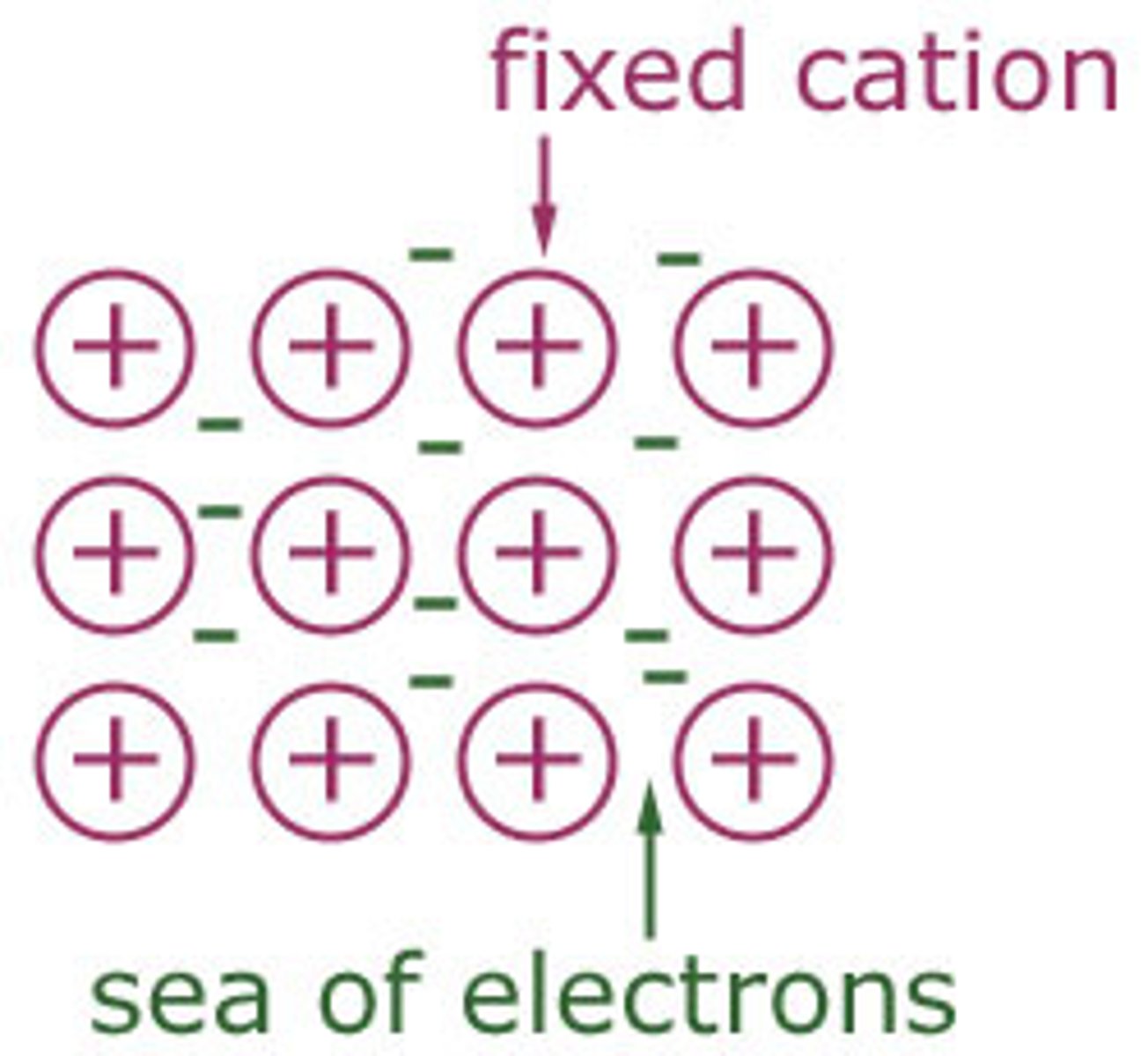
Why are electrons delocalised in the metallic structure?
Electrons are delocalised in metals because the valence electrons are not strongly bound to any individual atom due to them all being metals. They become free to move throughout the metal, forming a "sea of electrons" that binds the metal ions together.
What are the properties of metallic bonding
lustre, malleability, melting point, and electrical conductivity
Explain how metallic bonding accounts for the lustre, malleability, high melting point, and electrical conductivity of metals.
1. Metallic bonding creates a "sea" of delocalised electrons that reflect light, giving metals their lustre.
2. These electrons also allow metal ions to slide without breaking bonds, making metals malleable.
3. The strong attraction between metal ions and delocalised electrons causes high melting points.
4. Because electrons are free to move, metals conduct electricity well.
Strengths of the metallic bonding model
1. Explains key properties of metals
2. Simple and visual model
3. Predicts trends in metal reactivity, conductivity and strength
Limitations of the metallic bonding model
1. Oversimplifies electron behavior
2. Does not explain all metallic properties: magnetism
3. Limited for alloys and transition metals
4. Ignores atomic scale details
Describe how metallic character changes as you move down a group and across a period in the periodic table.
- Metallic character increases down a group due to the outer electrons being farther from the nucleus and easier to lose.
- It decreases across a period from left to right because increased nuclear charge holds electrons more tightly, reducing metallic behavior.
Explain how atomic structure causes the trend in metallic character down a group and across a period.
Metallic character increases down a group because more electron shells increase shielding and distance from the nucleus, making it easier to lose electrons.
Metallic character decreases across a period because increased nuclear charge pulls electrons closer, making them harder to lose.