Exam 4 Information
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
General information
- Meyer-Overton hypothesis —> refers to lipophilicity
- Mullins modification —> refers to lipophilicity and volumes
- GABAA —> enhances binding, leading to increased CNS depression:
E.g. propofol and midazolam
- Barbiturates increase GABA affinity to the receptor for depression
Stages of anesthesia
- Stage 1 - analgesia at low doses
- Stage 2 - excitement at increased doses (UNWANTED):
Causes amnesia (good), delirium, irregular respiration, and vomiting
- Stage 3 - surgical (WANTED):
Regular respiration —> wheeze regularly, lose consciousness, and pain insensitivity
- Stage 4 - medullary depression:
Respiration stops —> death
Ideal inhalation anesthetics
- Nonflammable
- Good chemical and metabolic stability
- Low incidence of MI effects
- Low incidence of hepatic and renal damage
- Rapid induction and emergence from anesthesia
- Adequate skeletal muscle relaxation
- Wide margin of safety
- Blood gas partition coefficients (BGPCs):
Enter lungs and distribute into the blood
Low (0.5) —> more in the gas phase, which means less in the blood, leading to a faster recovery
- Minimum alveolar concentration (MAC):
Low (1) —> only need 1% to produce activity in 50% (potent)
If >100, need 100% of dose to achieve the effect (low potency)
Nitrous oxide
- Gas at room temperature
- Good analgesic properties
- Weak anesthetic
- BGPC = 0.47 —> low so 2x as much in the gas phase
- MAC = 104% —> high so it’s weak and needs O2, so you can’t use 100%
- Always used in combination
- Very safe
- Fast onset
- Fast recovery because of low BGPC
- No hangover effect
- No skeletal muscle relaxation
Cyclopropane
- Gaseous anesthetic
- Produces anesthesia very quickly
- More potent —> very toxic
- When mixed with O2, it becomes flammable
- Stored in cylinders, which are heavy and large —> bad for storage
- Rarely used
- MAC = 17.5%
- Skeletal muscle relaxation
Ethylene
- Gaseous anesthetic
- Safer with less side effects
- No skeletal muscle relaxation
- MAC = 80%
Halothane
- Volatile liquid anesthetic
- Prototype fluorene general anesthetic —> derived from ethane
- When mixed with O2, it does NOT become flammable
- Increases molecular weight ineractions between molecules —> less volatile
- BP = 50.2 degrees Celsius —> liquid at room temperature
- BGPC = 2.5 —> favors the blood, so faster onset, but slower recovery
- MAC = 0.74% —> very potent and only need 0.74% of vapor in the airways
- Skeletal muscle relaxation
- Rapid induction and emergence
- 20% of the inhaled dose gets metabolized:
Metabolites are very toxic —> the ions can cause MI effects, as well as kidney and liver damage
Ketamine
- NMDA receptor antagonist —> allosteric (not the same site as glutamate)/noncompetitive (whether glutamate is bound or not, this drug will bind)
- Only basic center is the N
- Chiral center is on the C under the ketone —> S>R
- Racemic mixture
- Highly water soluble —> aqueous solution
- IV
- Produces dissociative anesthesia without loss of consciousness
- No skeletal muscle relaxation
- Extensive, rapid metabolism —> demethylation at the N (active metabolite —> norketamine), hydroxylation to the right of the ketone, and glucuronidation
- Short duration of action —> 10-25 minutes
- C-III substance
- Can produce hallucinogenic effects even 24 hours after recovering —> because of its similarity in structure to phencyclidine
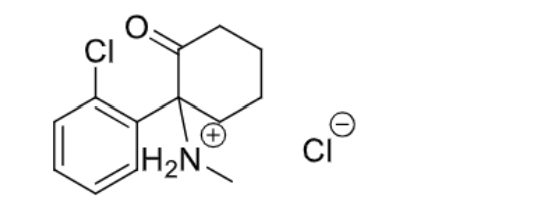
Esketamine
- Approved in 2019
- Nasal spray —> NOT IV
- Used to treat treatment-resistant depression
- Administered in the clinic under supervision of a HCP
- Used in combination with an anti-depressant
- 2x a week —> up to 84 mg
- Goes directly into the CNS —> faster onset
- C-III substance
Propofol
- NOT a controlled substance
- IV
- Works around GABAA, but the binding site is unknown
- Used for the induction and maintenance of anesthesia and for continuous sedation in adults in the ICU
- 40 second onset
- Faster recovery
- Anti-emetic properties
- Drug of choice for ambulatory surgery in outpatients
- Skeletal muscle relaxation
- Limited water solubility —> need to increase the pH and reduce the lipid emulsion to keep it in solution
- Can cause allergies and can harbor microbial growth
- Extensively metabolized via sulfation and glucuronidation
Fospropofol
- Attaches a phosphate group to a methylene group
- Prodrug —> gets metabolized by alkaline phosphatases
- Slower onset than propofol —> 4-8 minutes
- Not as successful as propofol —> less used now
- C-IV substance
Midazolam
- Imidazolo-benzodiazepine
- GABAA agonist —> more CNS inhibition
- Fluorine and chlorine substitution prevent metabolism
- Used for preoperative sedation and for induction of general anesthesia
- Slower onset than barbiturates —> <5 minutes for IV
- Prolonged CNS activity —> long recovery time
- Can cause amnesia and sedation
- Skeletal muscle relaxation
- Hydroxylation of the methyl on the imidazole ring
- C-IV substance
Remimazolam
- Benzodiazepine
- Soft drug —> the ester is active and it hydrolyzes via esterases to a carboxylic acid, making it more water-soluble and inactive
- Shorter duration of action compared to midazolam
- Approved in 2020
- The carboxylic acid is 300x less active at the GABAA receptor
- Used for short procedures <30 minutes
- Ultra fast-acting
- Skeletal muscle relaxation
- IV
- C-IV substance
Etomidate
- Soft drug —> the ester is active and it hydrolyzes via esterases to a carboxylic acid, making it more water-soluble and inactive
- Used to induce general anesthesia and treat patients in cardioversion —> short procedures
- IV
- No analgesic activity
- Fewer CV and respiratory depressive events
- Produces hypnosis within 1 minute
- Short duration of action —> 3-5 minutes
- Can cause N/V
- No skeletal muscle relaxation
- NOT a controlled substance
Methohexital
- Barbiturate
- Ultra-short acting —> <3 hours
- IV —> <20 minutes duration of action
- No skeletal muscle relaxation
- C-IV substance
- 2 chiral centers —> one on the C between the two ketones and one to the right of the triple bond
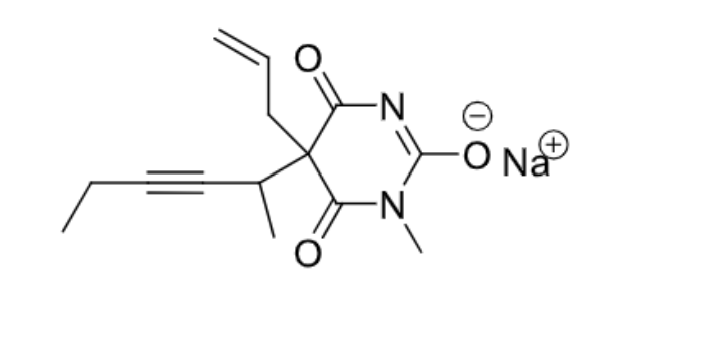
Thiopental
- Barbiturate
- C-III substance
- No skeletal muscle relaxation
- No unsaturation unlike methohexital
- The S is more lipophilic —> brings it into the CNS quicker and exits the CNS more easily
- Ultra-short acting
- The S can be removed and replaced with an O —> less lipophilic, short-acting (3-4 hours), and can cause long-term sedation
- Used to be the most commonly used because of its cheapness —> not the drug of choice now because of how it deposits into the fat
- Can cause prolonged anesthesia with repeated use
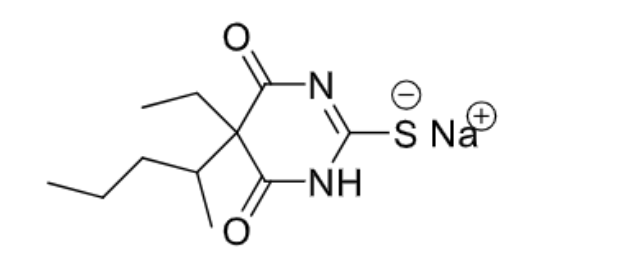
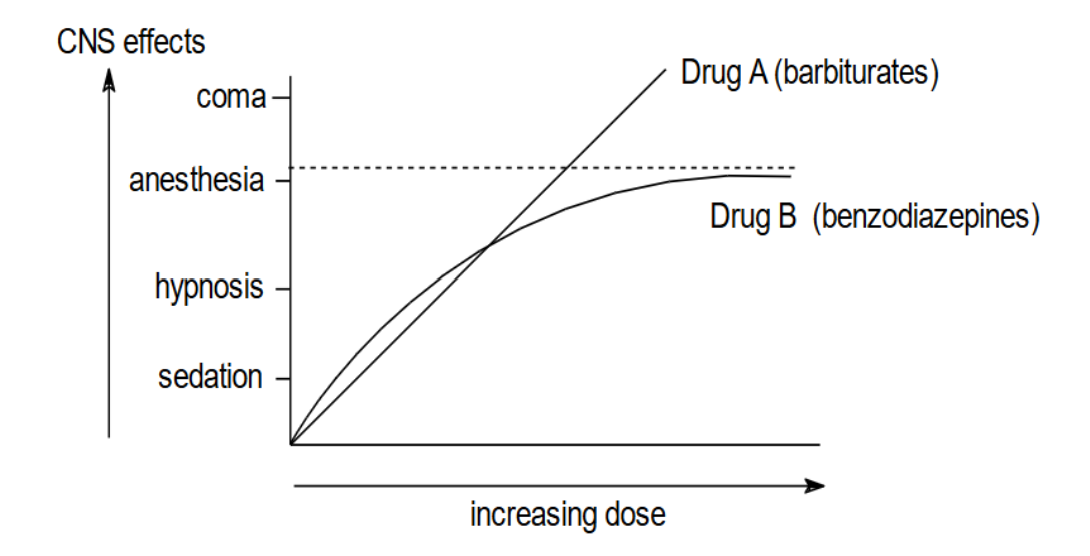
Barbiturates and benzodiazepines
- Benzodiazepines are safer than barbiturates
- GABAA and GABAB agonists
- Benzodiazepines have specific GABAA binding sites, whereas barbiturates do not
- GABAA —> post-synaptic inhibition
- GABAB —> pre-synaptic inhibition
Barbituric acid
- 2 imides —> 2 carbonyls between the N, making them acidic
- The CH2 between the 2 carbonyls is also acidic
- Tautomerization can occur
- O at the bottom right can be replaced with an S —> increases lipophilicity
- Highly water soluble
- In order to have CNS activity, the 2 hydrogens have to be removed from the CH2