chapter 10-11
1/104
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
105 Terms
What is a non-ferrous metal?
A metal, including alloys, that does not contain iron (ferrite) in appreciable amounts.
What are some unique properties of nonferrous metals?
Resistance to corrosion, ease of fabrication, high electrical and thermal conductivity, light weight, strength at elevated temperatures, and color.
What are the drawbacks of steel?
High density, low conductivity, and susceptibility to corrosion.
What is the backbone of the electrical industry?
Copper.
What are the general properties of copper?
High electrical and thermal conductivity, useful strength with high ductility, corrosion resistance, and low temperature properties.
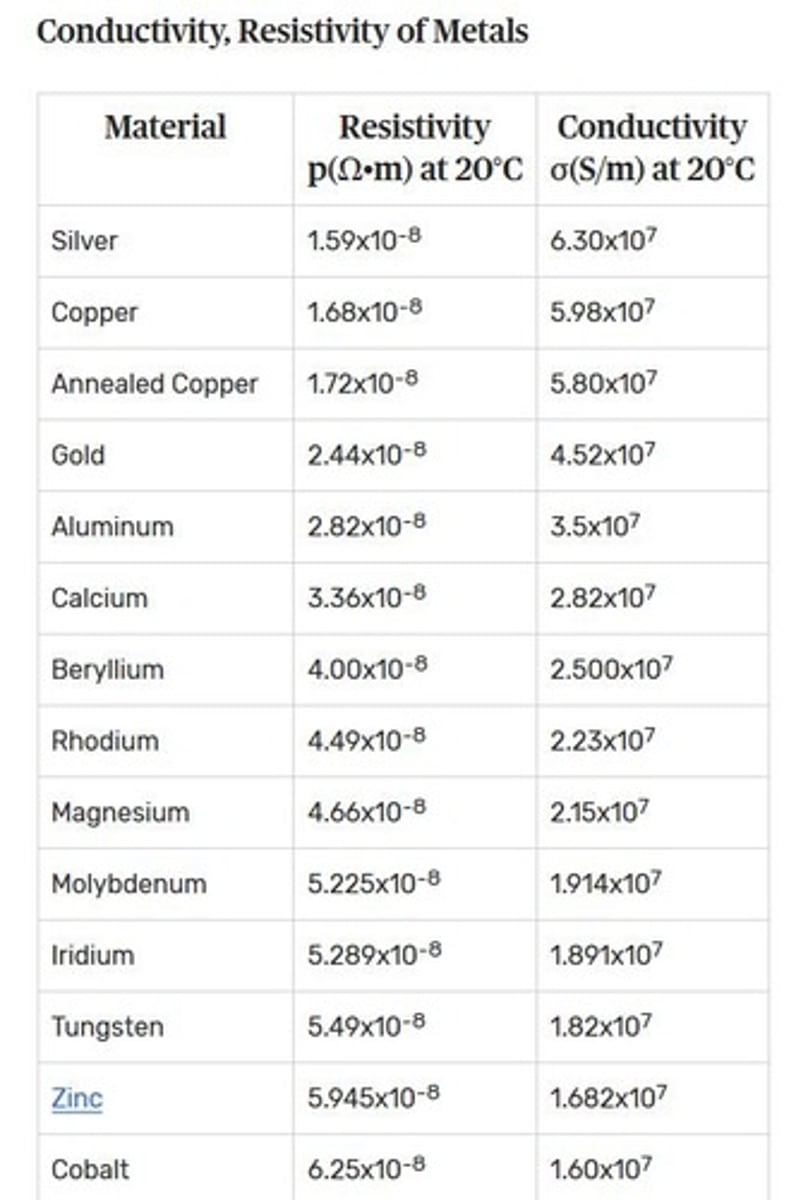
How does the strength of copper change with temperature?
Strength increases with decreasing temperature; it does not embrittle and retains ductility under cryogenic conditions. Conductivity increases with a drop of temperature.
(non magnetic, wide spectrum of colors
What are the common uses of copper?
Electrical applications, plumbing, heating, and air conditioning.
What are the drawbacks of copper?
Heavier than iron, problems at higher temperatures, and poor abrasive wear characteristics.
What are copper-based alloys?
Alloys where copper is the base metal, imparting ductility, corrosion resistance, and electrical and thermal conductivity.
What is the most common alloy addition to copper?
Zinc, known as brass.
(2nd tin, 3rd nickel)
What are alpha brasses?
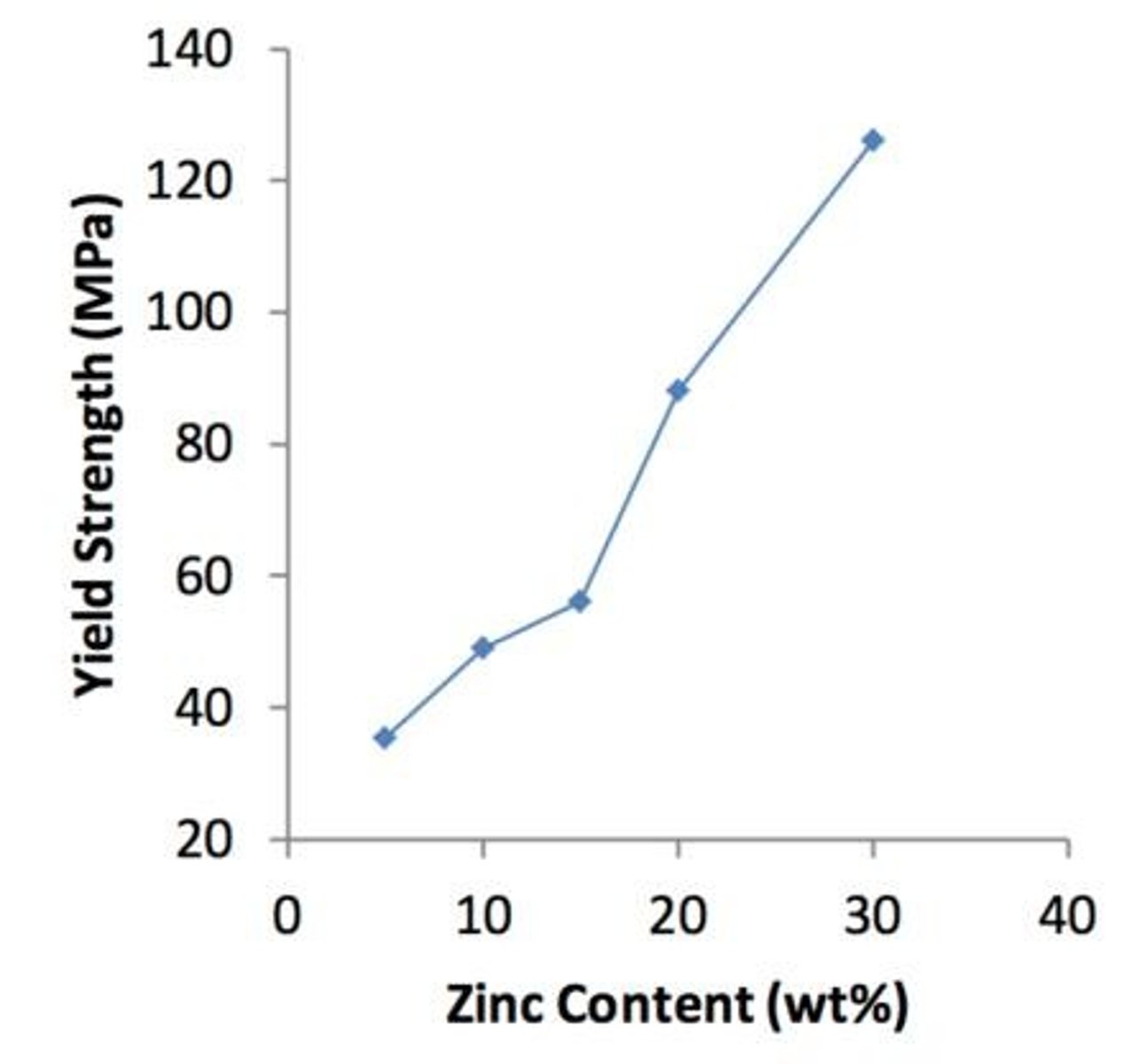
Ductile and formable copper-zinc alloys that increase in strength and ductility with increasing zinc content.
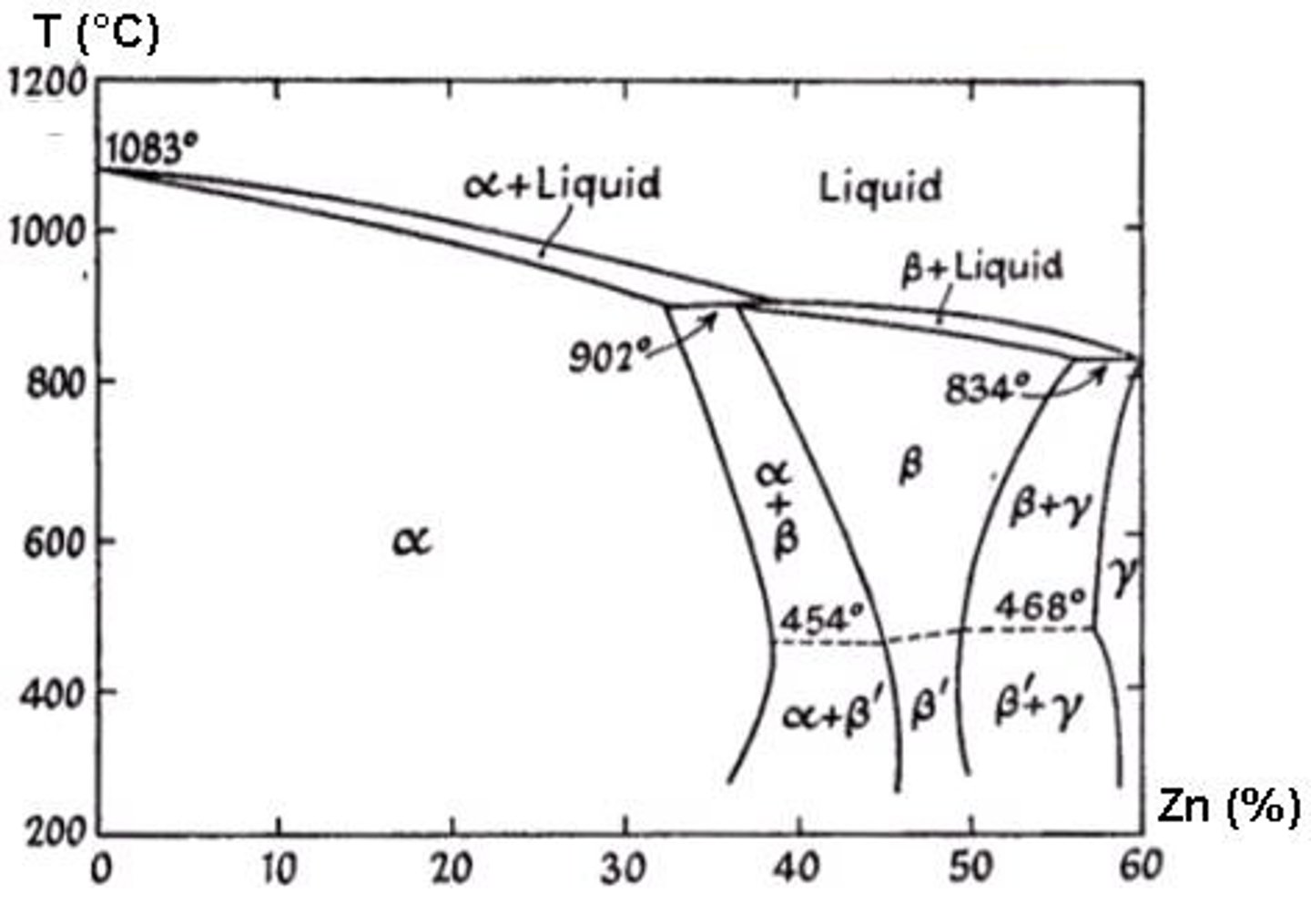
How can alpha brasses be strengthened?
By cold forming.
What are the characteristics of two-phase brasses?
High electrical and thermal conductivity, useful engineering strength, and a wide range of colors.
What happens to copper's conductivity as temperature decreases?
Conductivity increases with a drop in temperature.
What is a significant property of copper at low temperatures?
It retains ductility and does not embrittle.
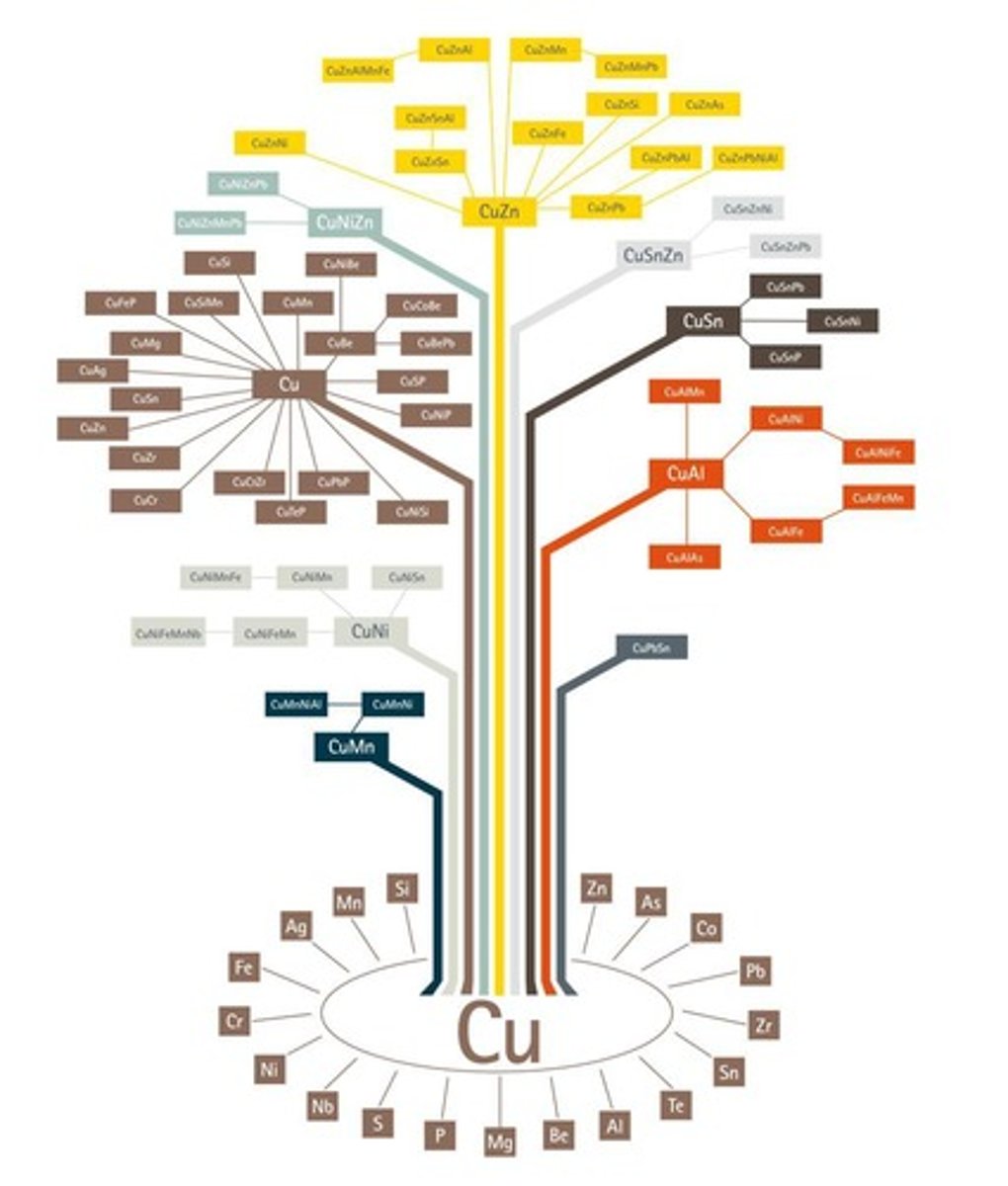
What is the significance of the copper alloy tree?
It shows the options for adding other metals to create different copper alloys.
What is a common application of copper in the electrical industry?
About one-third of copper is used in electrical applications.
What are the benefits of using copper in engineering?
Ductility, corrosion resistance, and high electrical and thermal conductivity.
What is the impact of increasing zinc content in brass?
Strength and ductility increase with increasing zinc content.
What is the color characteristic of copper and its alloys?
Copper and its alloys exhibit a wide spectrum of colors.
What are copper-tin alloys commonly known as?
Bronzes.
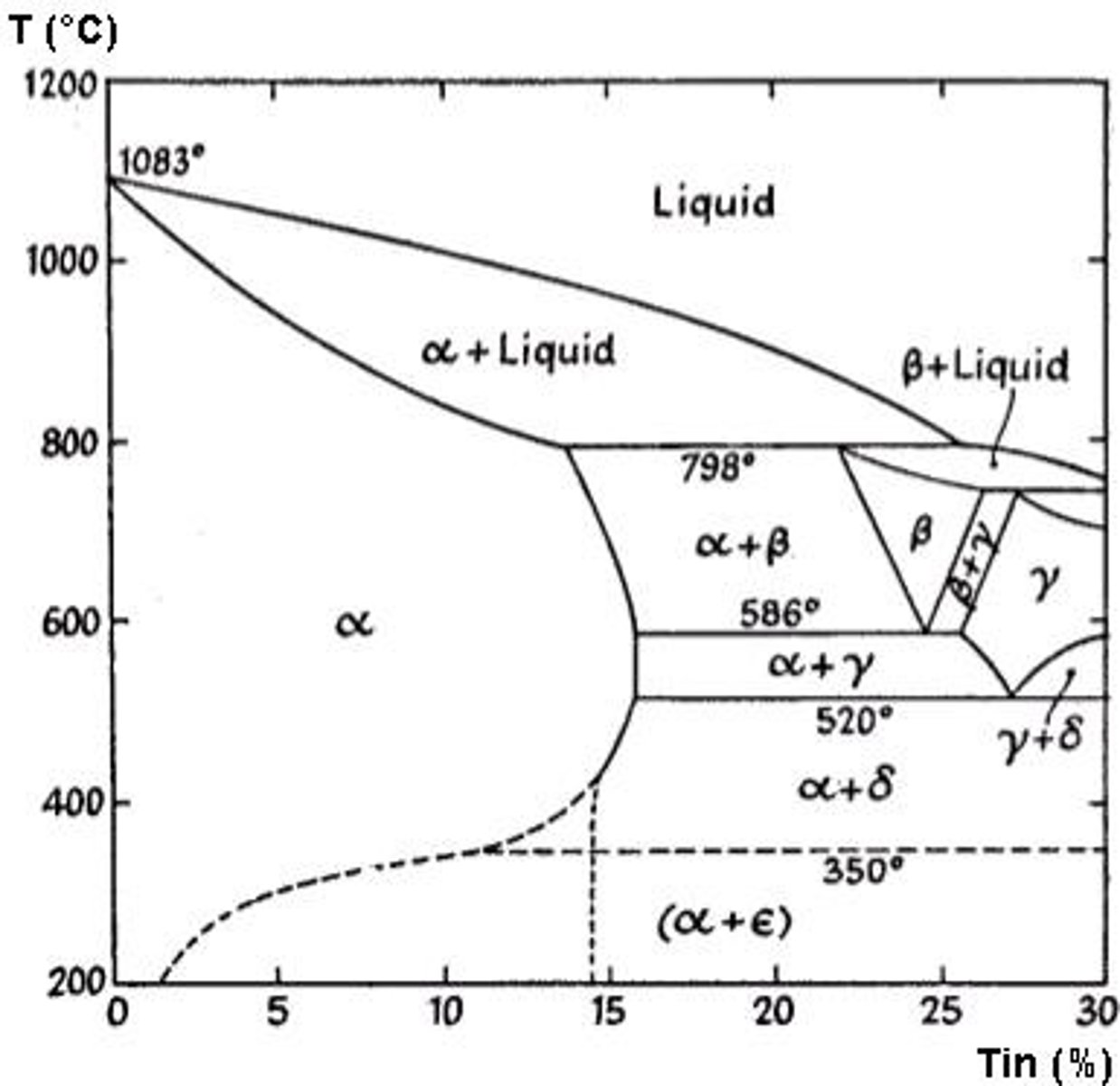
What is a key advantage of using tin in copper-tin alloys compared to zinc?
Tin is more cost effective than zinc.
What are some desirable mechanical properties of bronzes? (Copper tin)
Good strength, good toughness, good wear resistance, and good corrosion resistance.
What are common applications of copper-tin alloys?
Used for bearings, gears, and fittings with high compressive loads.
What is a notable characteristic of copper-nickel alloys?
Copper and nickel exhibit complete solubility.
High Thermal conductivity
High temperature
Corrosion resistance
High resistance to stress-corrosion cracking
Ideal as a heat exchanger
What are some properties of copper-nickel alloys?
High thermal conductivity, high temperature strength, corrosion resistance, and high resistance to stress-corrosion cracking.
What is the nickel content range in cupronickels?
2 to 30% nickel.
What is Monel?
An alloy that contains 67% nickel.
What are the typical alloying elements in aluminum alloys?
Copper, magnesium, manganese, silicon, tin, and zinc.
What are the general properties of aluminum?
Workable, lightweight, corrosion resistant, good thermal and electrical conductivity, optical reflectivity, and easily finished.
How does the weight of aluminum compare to steel?
Aluminum is about 1/3 the weight of steel for an equivalent volume.
What is a significant structural limitation of aluminum alloys?
Lower fatigue strength compared to steel.
In what applications are aluminum and its alloys commonly used?
Transportation, packaging, containers, and building construction.
What are the advantages of aluminum as a material for aircraft structures?
Moderate cost, ease of fabrication, excellent corrosion resistance, lightweight, high specific strength and stiffness, good ductility and fracture toughness.
What is a drawback of aluminum alloys at elevated temperatures?
Low mechanical properties at temperatures above ~150°C.
What are the two principal classifications of aluminum alloys?
Cast alloys and wrought alloys.
What is a notable difference between casting alloys and wrought alloys?
Mechanical properties of casting alloys are generally inferior to wrought alloys.
In what situations are casting alloys typically used?
Sometimes used in small, non-load-bearing components.
What percentage of composites, including CNT, is used in the F35?
35%.
What alloying elements are commonly found in commercial aluminum alloys?
Cu, Si, Mg, Mn, and occasionally Zn, Ni, and Cr.
Which can enhance mechanical properties by solid solution hardening, strain hardening by cold work, responding to precipitation hardening
What does the 'F' designation indicate in aluminum alloys?
Fabricated.
What does the 'H' designation indicate in aluminum alloys?
Strain hardened.
What does the 'O' designation indicate in aluminum alloys?
Annealed.
What does the 'T' designation indicate in aluminum alloys?
Thermally treated.
What does the 'W' designation indicate in aluminum alloys?
Solution-heat-treated only.
What is solid solution hardening?
A process that enhances mechanical properties by dissolving alloying elements in the base metal.
What are the characteristics of 2XXX aluminum alloys?
Al Cu alloys that can be precipitation hardened to strengths comparable to steel, formerly known as duralumin.
What are the characteristics of 3XXX aluminum alloys?
Al Mn alloys that are not heat-treatable.
What are the characteristics of 4XXX aluminum alloys?
Al Si alloys that are not heat-treatable.
What are the characteristics of 5XXX aluminum alloys?
Al Mg alloys that are not heat-treatable.
What are the characteristics of 6XXX aluminum alloys?
Al Si-Mg alloys that are easy to machine, weldable, and can be precipitation hardened, but not to the high strengths of 2000 and 7000 series.
What is the most commonly used general-purpose aluminum alloy?
6061 alloy.
What are the characteristics of 7XXX aluminum alloys?
Al Zn alloys that can be precipitation hardened to the highest strengths of any aluminum alloy.
What does the second digit in the aluminum alloy designation indicate?
Modifications to the alloy.
What is the significance of the last two digits in aluminum alloy designations?
They have no meaningful relationship to the composition; they identify different alloys in a series.
What are some applications of nickel-based superalloys?
Aircraft gas turbines, steam turbine power plants, medical applications, nuclear power systems, and chemical and petrochemical industries.
What distinguishes a superalloy from a regular alloy?
A superalloy is a high-performance alloy resistant to high temperatures, while an alloy is a combination of two or more elements, at least one being a metal.
What mechanical properties do nickel-based superalloys provide at high temperatures?
Improved creep strength, resistance to corrosion or oxidation, and mechanical strength.
What is a notable application of nickel-based superalloys?
Components in compressors, combustion chambers, turbine components, exhausts, and nozzles.
How do nickel-based superalloys compare to other structural materials at high temperatures?
Nickel-based superalloys maintain mechanical properties like yield and ultimate strength, while materials like carbon steel and magnesium lose these properties.
What is the carbon content of plain carbon steel?
Low carbon steel
C < 0.25%
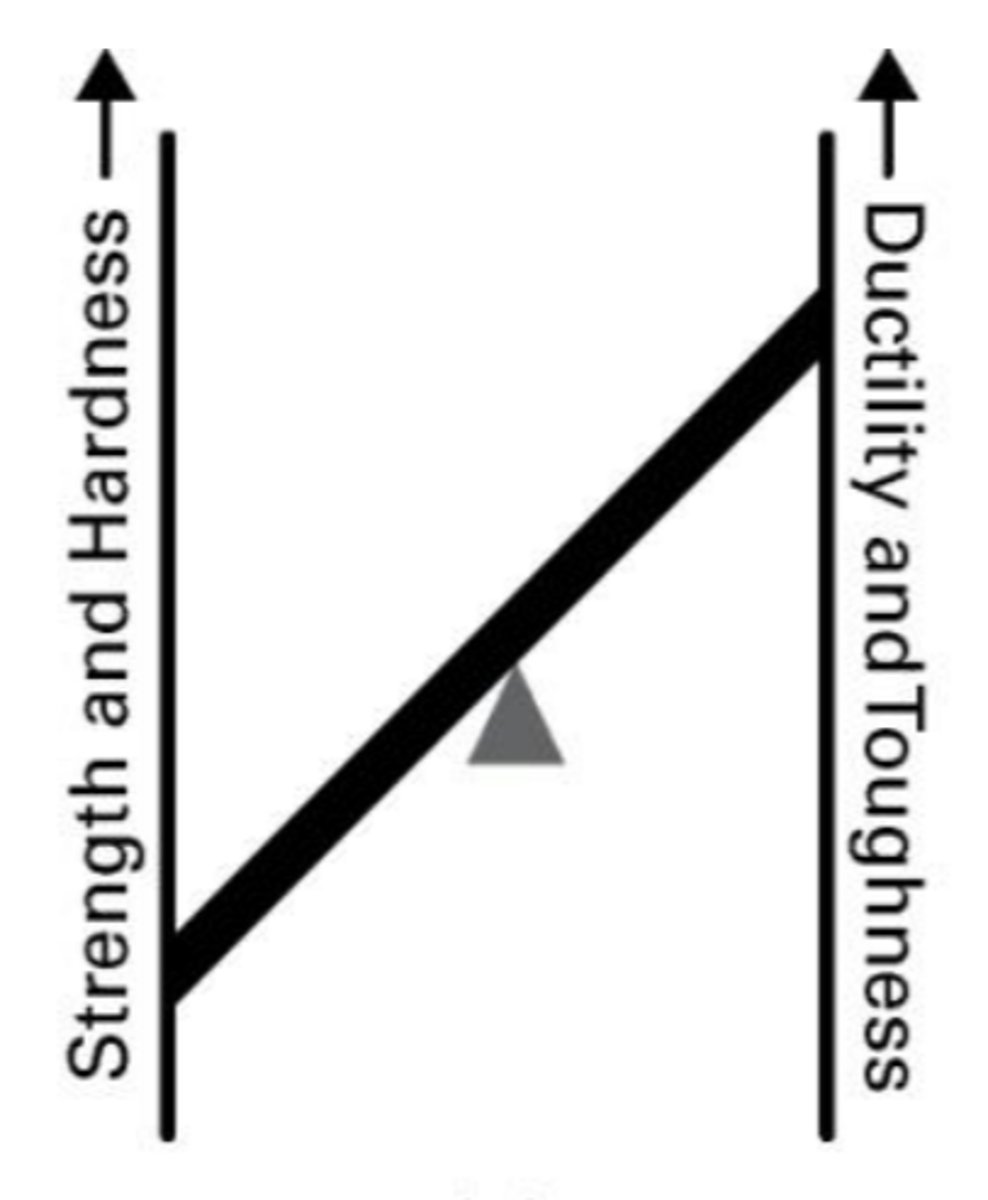
What are the key properties of plain carbon steel (low carbon steel)?
Good ductility, toughness, weldability, and machinability. Sheet metal forming.
What is the carbon content range for median-strength carbon steel?
0.25% < C < 0.60%
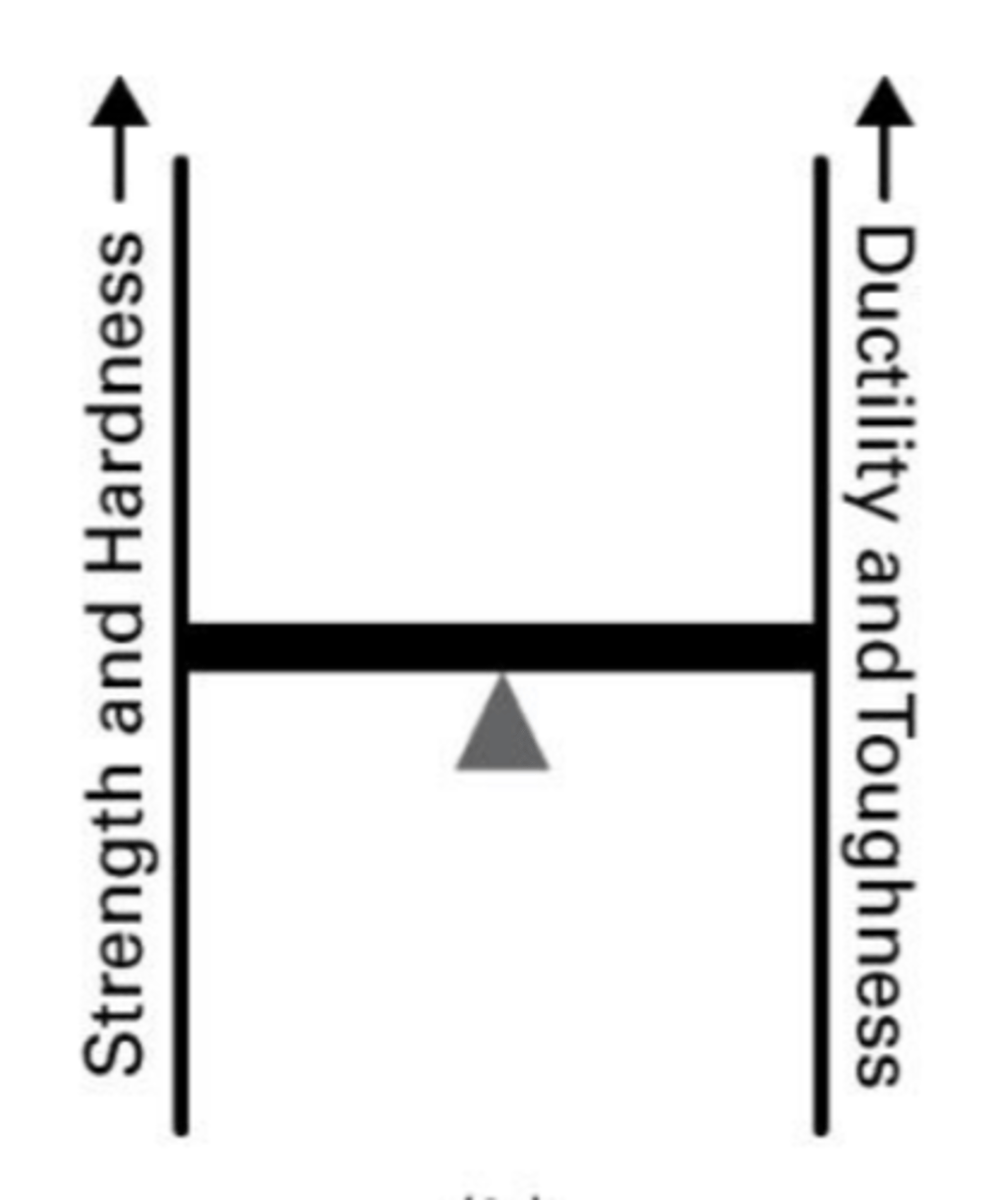
What is a common application and properties for median strength carbon steel?
Automotive parts.
high strength , poor ductility
What is the carbon content range for cutting tool steel?
hint: hig strength poor ductility
0.6% < C < 2.1%
(high carbon steel)
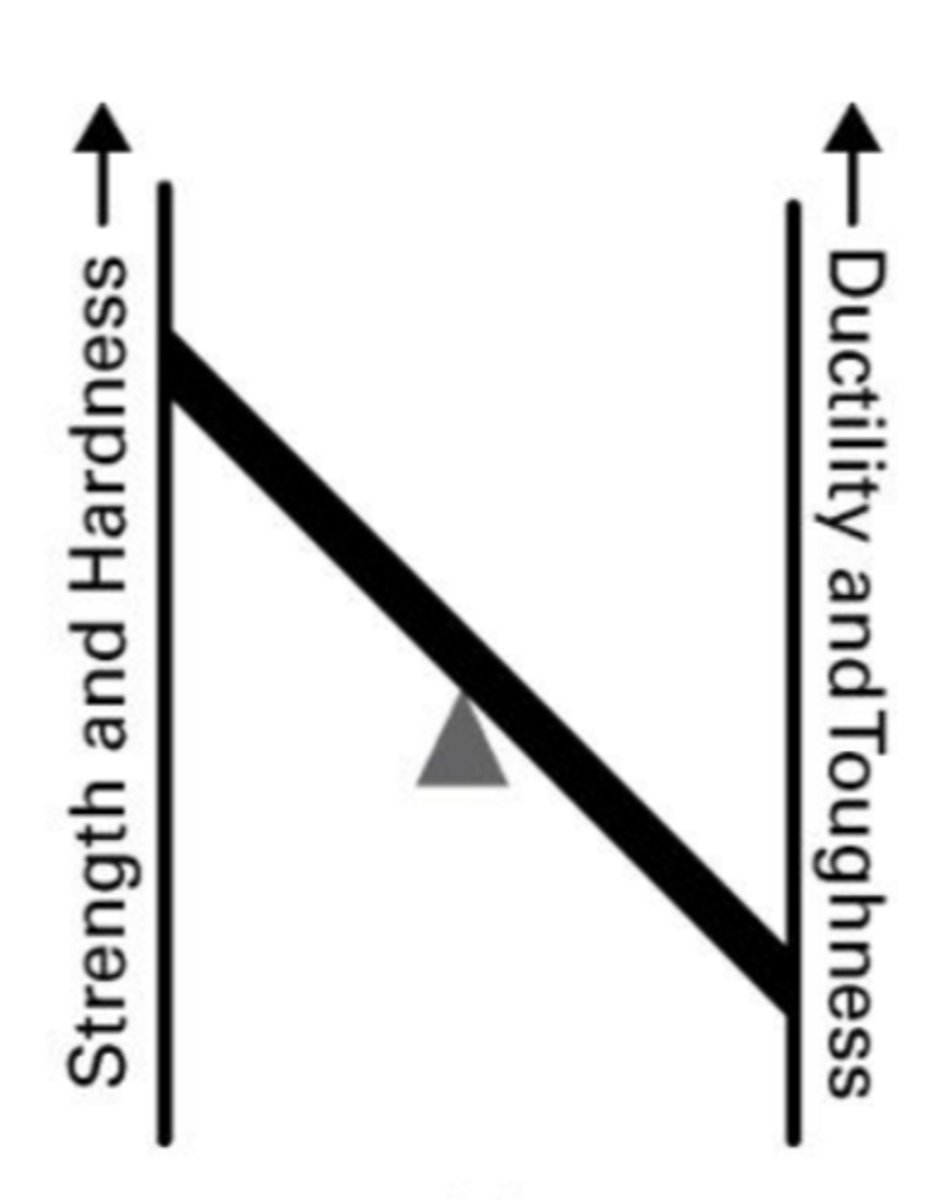
What defines alloy steel?
Steels containing alloys in specifiable amounts:
1.65% or more maganese
0.6 % silicon
0.60 % copper
What are the most common alloying elements in alloy steel?
Chromium, nickel, molybdenum, vanadium, tungsten, cobalt, boron, and copper.
What is the distinction between low alloy and high alloy steels?
Low alloy steels contain less than 8% alloy additions
High alloy steels contain more than 8% alloy additions.
Why is iron considered the most important engineering metal?
It is one of the four most plentiful elements in the earth's crust.
In what form does iron typically occur in nature?
In a variety of mineral compounds known as ores, such as magnetite (Fe3O4) and hematite (Fe2O3).
What is the iron content of magnetite?
72.4% Fe.
What is the iron content of hematite?
69.9% Fe.
What is metallic iron made from?
Processing iron ore.
What does the processing of iron ore break?
Iron-oxygen bonds.
What materials are continuously inputted into a furnace to extract molten metal?
Ore, limestone, coke (carbon), and air.
What is the product of extracting molten metal from iron ore?
Pig iron.
How is steel manufactured from pig iron?
By an oxidation process that decreases carbon, silicon, manganese, phosphorous, and sulfur.
What principle does a blast furnace operate on?
Chemical reduction.
What are the two main processes for manufacturing steel?
Kelly-Bessemer process and Open-hearth process.
What must steel undergo to transition from liquid to solid?
Solidification.
What does continuous casting produce?
Feedstock material used in forging or rolling operations.
What is ladle metallurgy designed to provide?
Final purification, alloy additions, reduction or removal of dissolved gases, and grain size refinement.
What is the carbon content range for High-Strength Low-Alloy Structural Steels (HSLA)?
0.05-0.25%.
Why are HSLA steels not considered alloy steels in the normal sense?
They are designed to meet specific mechanical properties rather than a chemical composition.
What are some alloying elements used in HSLA steels?
Manganese (up to 2.0%), copper, nickel, niobium, nitrogen, vanadium, chromium, molybdenum, titanium, calcium, rare earth elements, or zirconium.
What is the microstructure of Dual-phase steels (DPS)?
A combination of ferrite and martensitic microstructure.
How is Dual-phase steel produced?
Starts as low or medium carbon steel and is quenched from a temperature above A1 but below A3.
What is the advantage of HSLA steels compared to carbon steel?
Better mechanical properties (strength-to-weight ratio) and greater resistance to corrosion.
What is the purpose of pouring processed liquid into a continuous caster?
To create solid steel products.
What processes can ladle metallurgy perform?
Final purification, alloy additions, reduction of dissolved gases, and grain size refinement.
What is the significance of the continuous cooling transformation diagram in steel processing?
It guides the quenching process for producing Dual-phase steels.
What happens to the molten metal during the steel manufacturing process?
It is poured into ladles for further processing.
What is the role of coke in the furnace during iron production?
It acts as a source of carbon and helps in the reduction of iron ore.
What is the microstructure of alloy steels composed of?
A soft ferrite matrix containing islands of martensite as the secondary phase.
How does martensite affect the properties of alloy steels?
Martensite increases the tensile strength.
What is the microstructure composition of Transformation-Induced Plasticity (TRIP) steel?
Islands of hard residual austenite and carbide-free bainite dispersed in a soft ferritic matrix.
What happens to austenite during plastic deformation in TRIP steels?
Austenite is transformed into martensite.
What is the significance of the Transformation Induced Plasticity (TRIP) effect?
It allows for greater elongations and provides an excellent combination of strength and ductility.
How does the carbon content in TRIP steels compare to dual-phase steels?
TRIP steels use higher quantities of carbon to stabilize the retained austenite phase below ambient temperature.
What is required for TRIP steels to achieve their microstructure?
An isothermal hold at an intermediate temperature, which produces some bainite.