hydrate, hemiacetals, and acetals
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
hydrate
geminal diol, contains two OH groups
acidic reaction conditions
positive or neutral intermediates only, strongest acid is H3O, strongest base is water
basic reaction conditions
negative or neutral intermediates only, strongest acid is water, strongest base is OH
how will carbonyls form hydrates under acidic conditions?
H3O will protonate O forming a pos charge, water will attack C at double bond and break to remove pos charge, another molecule of water will deprotonate remaining positive oxygen
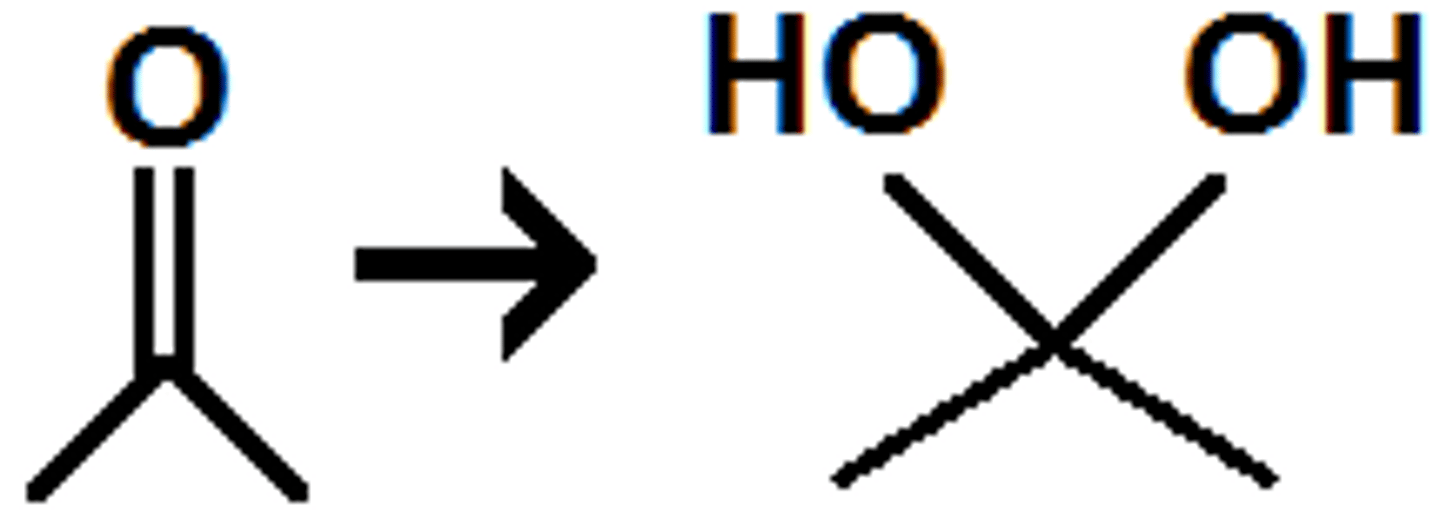
how will carbonyls form hydrates under basic conditions?
OH will attach C at carbonyl, breaking double bond forming neg O, water will protonate neg O
hemiacetal and hemiketal
carbonyl analog with one OH and one OR group
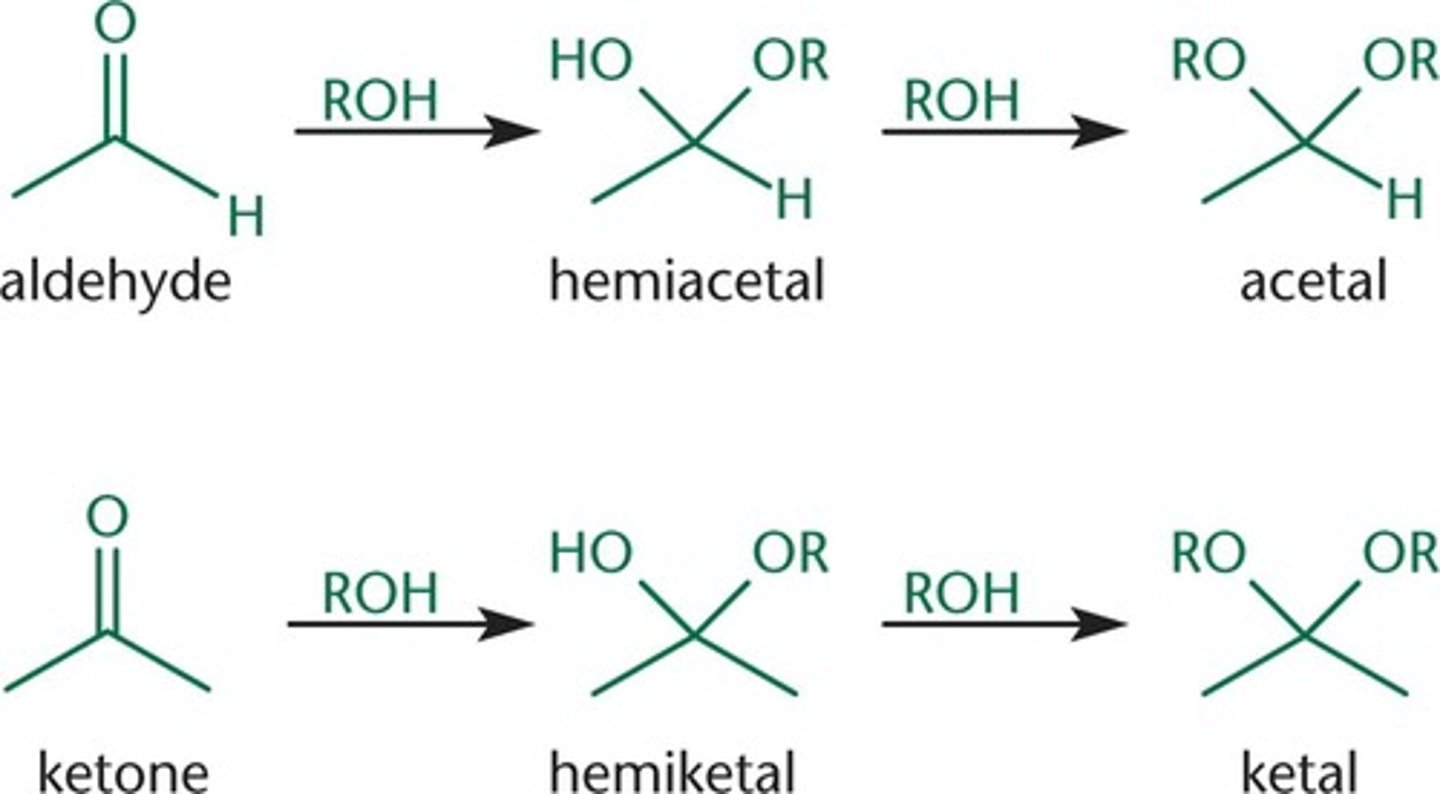
when do cyclic hemiketals form?
when the solvent used leads to equilibrium being favored in the cyclic form
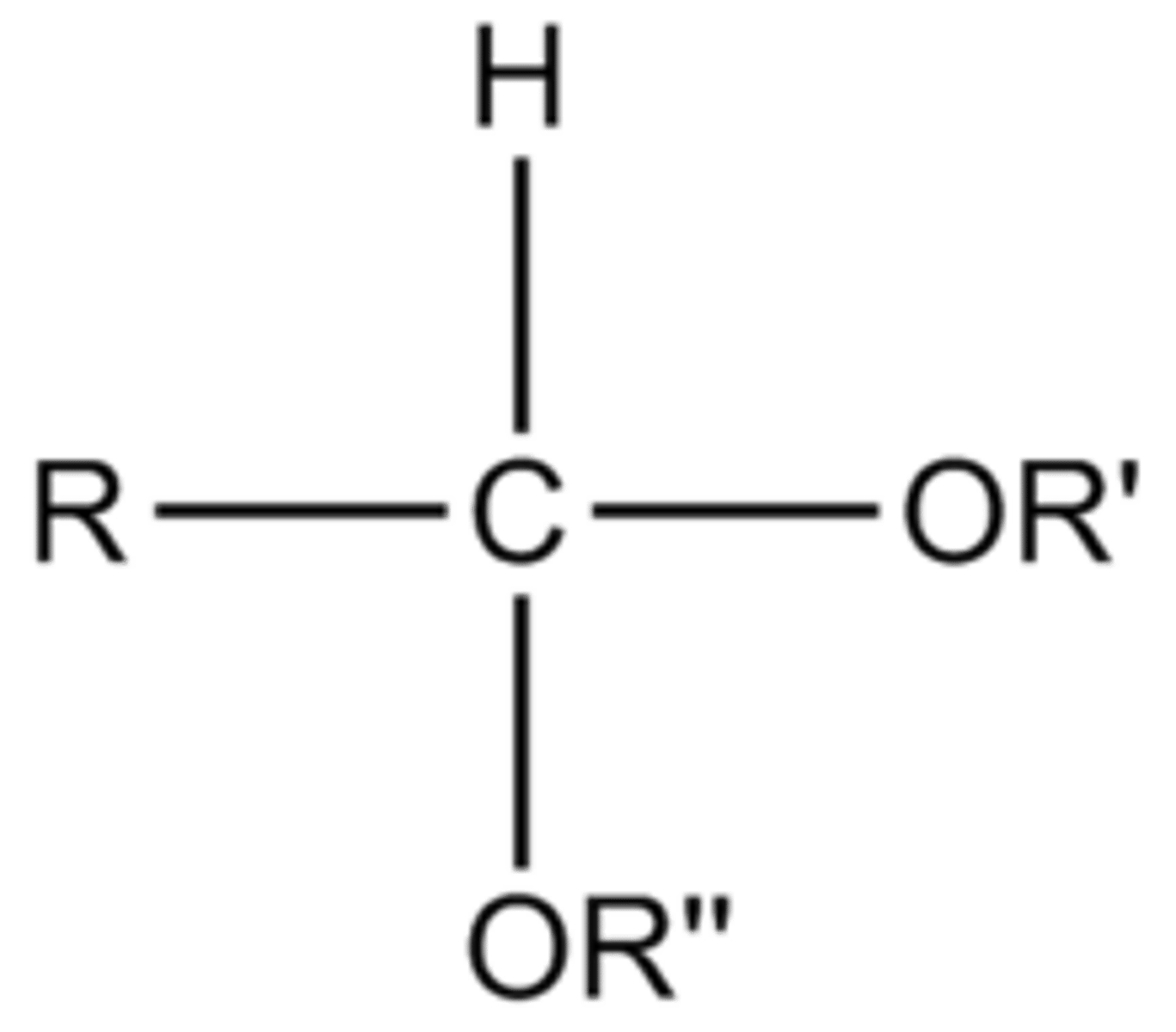
H+/2 eq R-OH
acetal formation from an aldehyde or ketone using R group from alcohol, 8 step mechanism with hemiacetal being formed at the 4th step
what are the usual alcohols being used to form acetals?
pTsOH, strong acid and soluble in organic compounds
what condition can acetals not be formed under?
basic or neutral
p-TsOH/diol
aldehyde or ketone to cyclic acetal (double bond split to form ring)
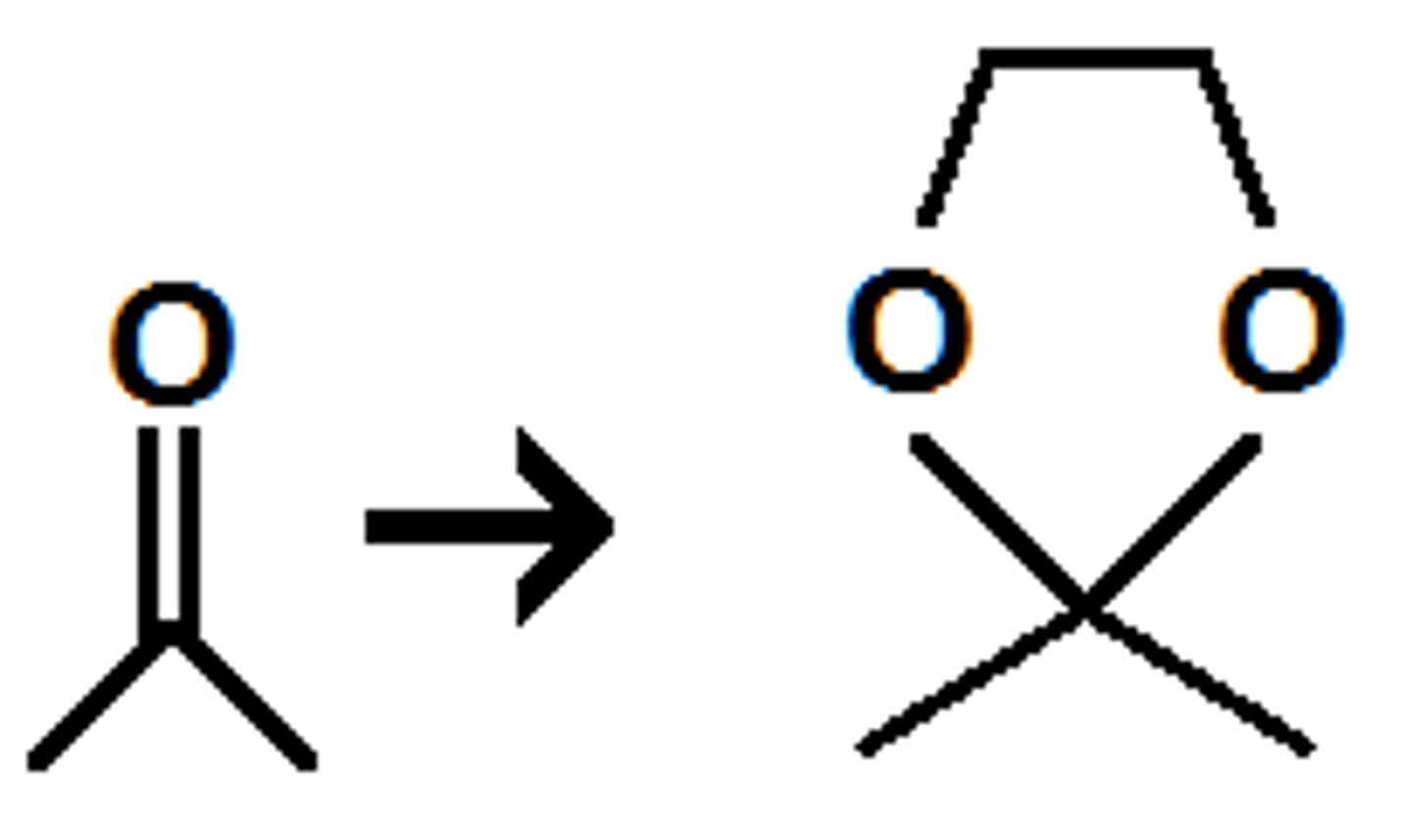
how do you revert cyclic acetals back to carbonyls?
hydrolysis (H3O)
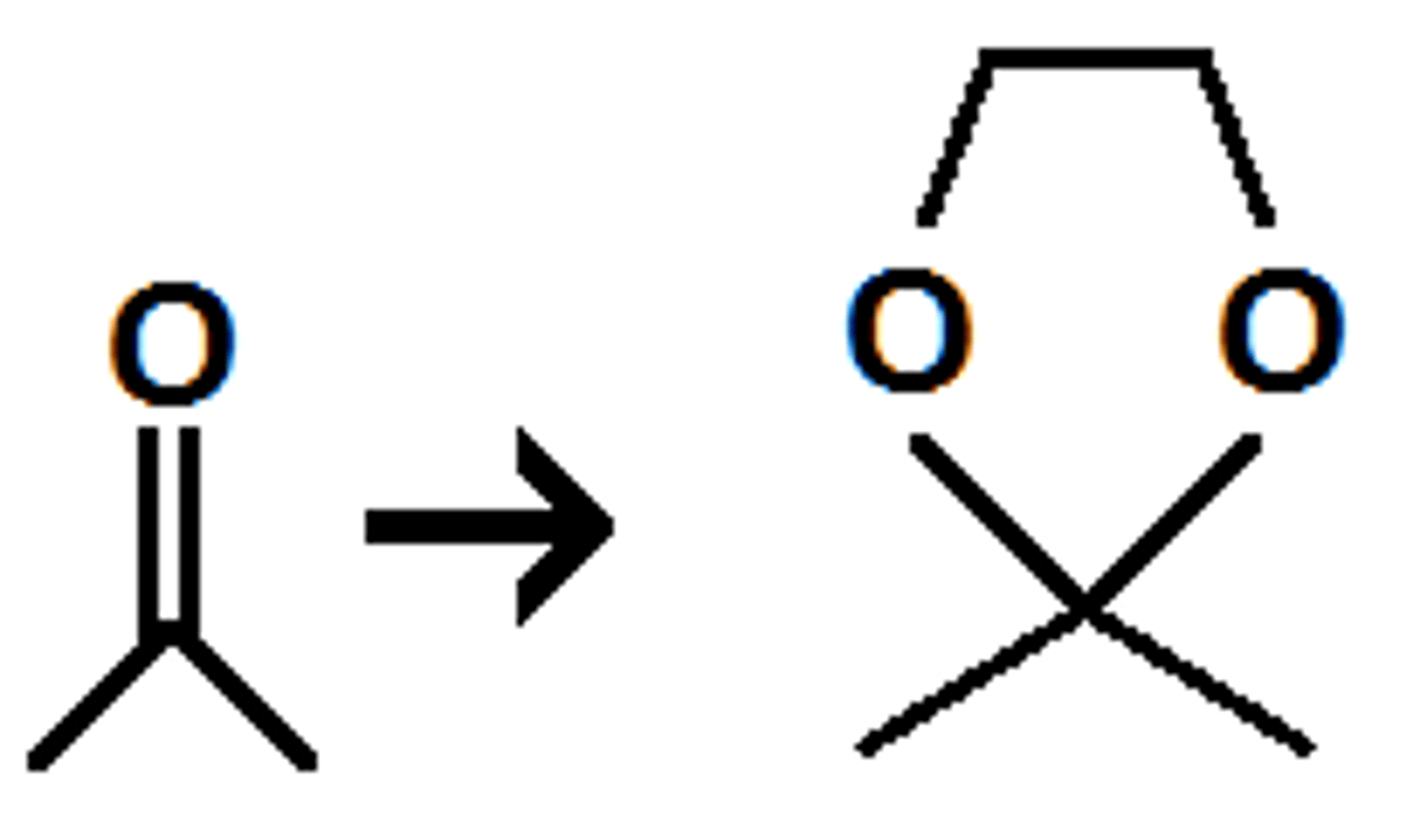
what is the best synthetic use for cyclic acetal formation?
to create a protecting group for OH molecules in organometallic reactions
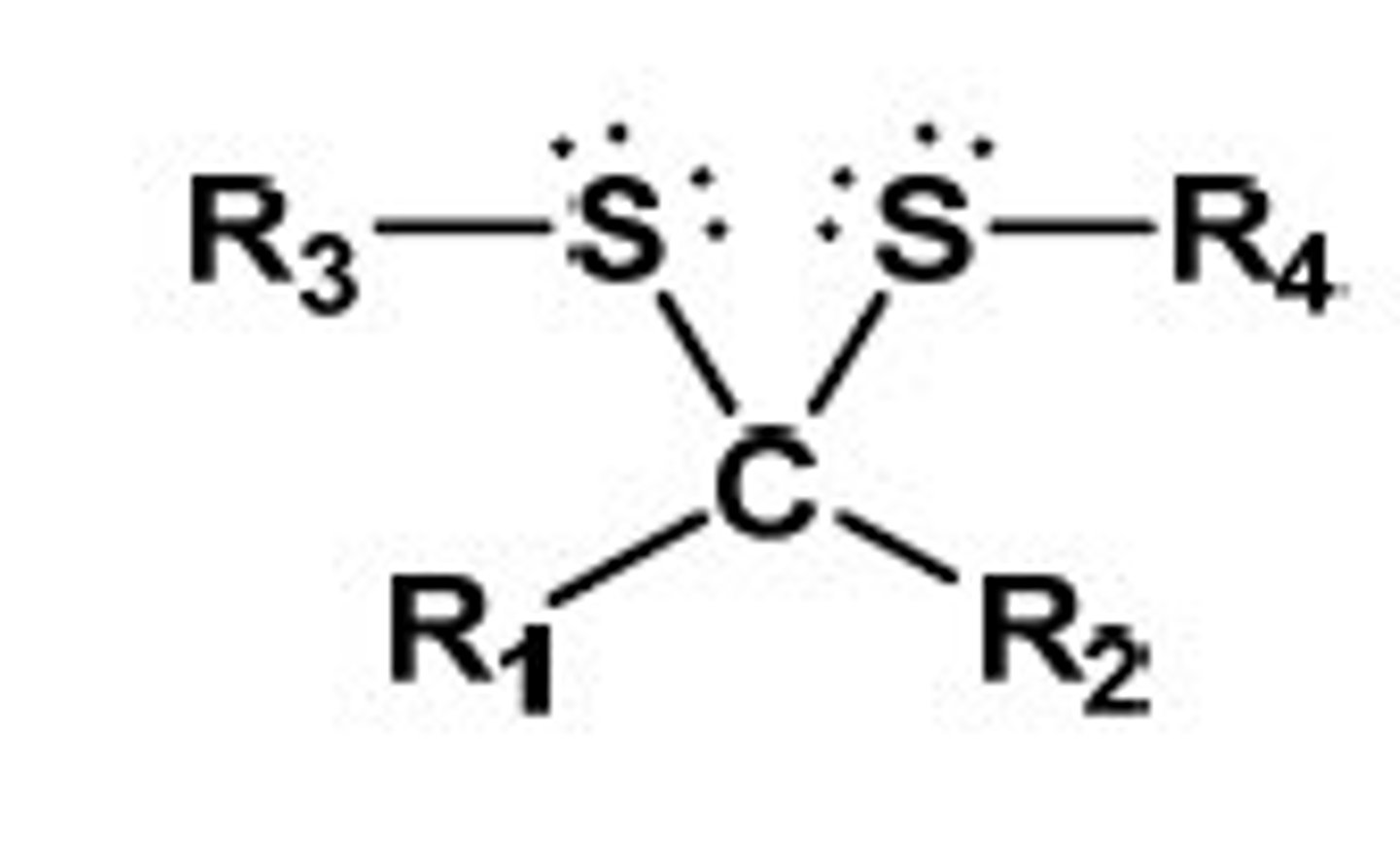
p-TsOH/ 2 eq R-SH
aldehyde or ketone to diothioacetals
BF3/dithiol
aldehyde or ketone to cyclic dithioacetals
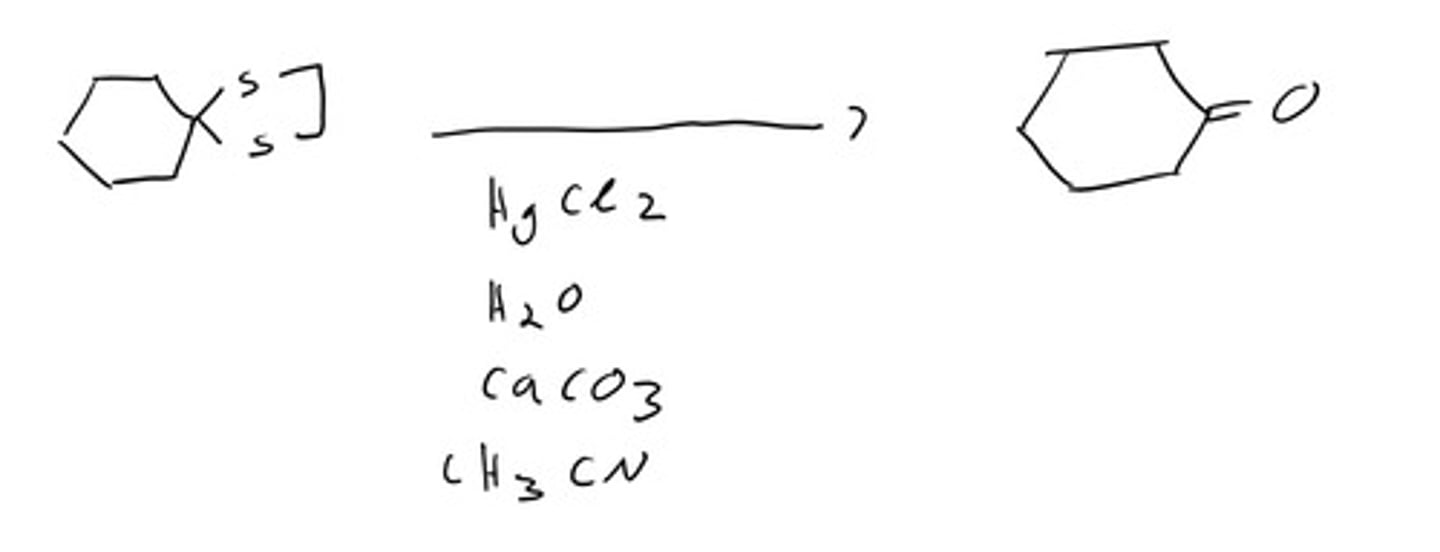
H2/Raney Ni
dithioacetal to alkane, will also reduce alkenes and alkynes
carbonyl to alkane
form dithioacetal from carbonyl and reduce with Raney nickel