1.8 - Electrochemistry
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
Why does Zinc become aqueous in the following reaction:
Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Zinc becomes an aqueous ion more easily as less energy is needed for atomisation + ionisation
What dictates if a Metals ability to displace another metal in a reaction?
Reduction strength:
The metal that displaces the other is a greater reducing agent, but weaker oxidising agent
How do you know if a Metal will reduce or oxidise another based off the Electrochemical series?
Based on the “order” of reduction/oxidising power:
Rule of thumb = Anything below a reducing/oxidising agent will be affected in the same way
Basically the more reducing agent = more -ve electrode potential
What is a Half Cell?
One half of an electrochemical cell, formed from a metal electrode dipped in its ions, or a platinum electrode with 2 aqueous ions
When is a Platinum electrode used? (2)
When a solution has 2 or more aqueous ions
When the element may be in gaseous form
Why use a Platinum electrode? (2)
Chemically inert
Electrically conductive
Why do we separate Half Cells?
So that the less reducing metal half cell doesn’t gain electrons + ensure electron flow to be usable → electricity
What is an Electrochemical cell?
Cell made of 2 half cells joined by a wire, voltmeter + salt bridge
What is a Voltmeter used for?
Measures PD between 2 half cells
How do electrons flow in an Electrochemical cell?
Electrons flow from a more reactive metal to a less reactive metal
Where are the +ve and -ve electrodes placed in an Electrochemical cell?
Why
+ve = Right
-ve = Left
Since electrons flow from negative to positive charges
Where does equillibrium lie to in:
+ve elecrode
-ve electrode
Positive = More reducing → Equilibrium lies left (more ions)
Negative = More oxidising → Equilibrium lies right (more atoms)
What is the Acronym for identifying which electrode is Oxidising + Reducing?
NOR PRoblem:
N.O.R = Negative → Oxidises itself (reduces solution) → (Reverse when combining half cell equations)
P.R = Positive → Reduces itself (oxidises solutions)
Describe the distribution of charge or electrons in an Electrochemical cell
Negative = Since the (more) reducing gains electron charge builds up on its electrode. We connect a wire to the more oxidising electrode + electrons flow from the negative electrode to the other. The number of electrons in the reducing agent decreases to maintain equilibrium.
Positive = The more oxidising electrode (more negative electrode) atoms revert to aqueous ions. The number of electrons increase for the more reducing electrode (more positive electrode), therefore to oppose the change, equilibrium shifts right so more ions in solution convert to atoms.
What is the equation for the EMF of an Electrochemical cell?
Ecell = Ereduced - Eoxidised
What is the following answers to the Electrochemical series: (why)
Most reducing agent
Most oxidising agent
Most reducing = F2 (most electronegative)
Most oxidising = Sr (least electronegative)
What is the Standard Hydrogen Electrode?
Why use Hydrogen gas? (2)
Why use that specific electrode? (2)
Where gaseous Hydrogen is bubbled into a half cell containing 1 moldm^-3 of H+ ions in solution, a platinum electrode = 0.00V
Hydrogen is abundant + cheap
Inert + Conducts electricity
What are the 3 Standard Conditions of S.H.E?
Diprotic acid
298K
100kPa
1 moldm-3 of H+ ions -> 1 moldm-3 of HCl or 0.5 moldm-3 of H2SO4 (since its diprotic)
How does equilibrium shift change EMF?
Same Le Chateliers principle changes:
Makes EMF more negative = More electrons
Makes EMF more positive = Less electrons
Cells
Cells
Give the following:
Electrode polarity
What occurs at the electrode
What type of agent is it
Negative electrode → Undergoes Oxidation → Is the Reducing agent
Positive electrode → Undergoes Reduction → Is the Oxidising agent
Explain how dynamic equilibrium in a Cell is met
Include mention of movement of electrons
Solution charges
(key one correct understanding)
The more reducing agent (negative electrode) loses electrons as electrons flow between electrodes via a wire, decreasing the number of electrons causing an equilibrium shift to the left to increase the number of +ve ions → solution of the negative electrode is more +ve
The more oxidising agent (positive electrode) gains electrons from the negative electrode via a wire, increasing the number of electrons and therfore reacting with ions to form more atoms → decreasing the concentration of +ve ions decreases the charge of the solution making the solution more -ve
What causes an EMF to drop?
Lack of ions in solution prevent the flow of electrons, causing charge build up → dropping PD
How do we prevent the build up of charge (decrease PD)
How does it work?
Adding anions (-ve ions) to the +ve solution (the negative electrodes solution) and cations (+ve ions) to the -ve solution (the positive electrodes solution) to allow the flow of ions to prevent charge build up
Prevention = Salt bridge
What is a Salt Bridge?
Filter paper soaked in saturated ionic solution that contains (cat/an)ions, to allow for ion flow to balance charges
What are 3 causes that lead to PD drop?
All of an electrode has been turned to aqueous ionised
All of aqueous ions form atoms on the electrode surface
When the ions of a salt bridge are depleted
What is a commonly used solution for Salt bridges?
Why (2)
KNO3:
It contains (cat/an)ions
Ions dont react with metal ions in their solutions
What 4 ions aren’t used for Salt Bridges?
Why
Halides
Hydroxides (OH-)
S2-
CO3-
All form precipitates which coat an electrode → slowing the electron transfer + drops EMF
What is the Layout for Cell Notation
Layout
Symbol meanings
Order
Rule (use example if needed)
Reduced Form | Oxidised Form || Oxidised Form | Reduced Form
" | " = State difference
" || " = Salt bridge
" , " = Separating DIFFERENT ions in the SAME
Oxidised form = “ X | X+, X2+ || Y2+, Y+ | Y”, where the more oxidised metal is closer to the salt bridge → not the more +ve ion
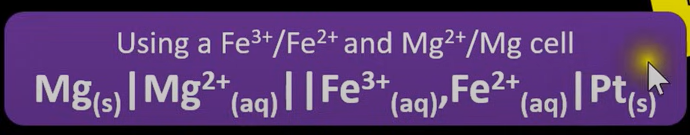
When do we know if EMF is feasible?
+ve PD value
Non-Rechargeable cells + Commercial applications
Non-Rechargeable cells + Commercial applications
What is the rule when combining half cell equations?
When combining equations, use LCM to get the same number of electrons, then cancelling them out
***USE CHEM NOTES 1 + BLURT 2 FOR DIAGRAMS AND OTHER BATTERY TYPES***
***USE CHEM NOTES 1 + BLURT 2 FOR DIAGRAMS AND OTHER BATTERY TYPES***
What is a Separator?
A porous material that separates +ve and -ve solutions to prevent an instantaneous redox → causing an explosion, allowing for the flow of ions between solutions to prevent charge build up
What is a Dry cell?
Advantage
A cell where reactants have moisture, but not entirely, to allow the flow of ions but decrease mass and size:
Prevents loss of reactants via spillage
What is an Electrolyte?
3 examples
Solution or paste with free moving ions allowing flow of electron in a circuit:
ZnSO4
Fe2(SO4)3
MgSO4
How do we recharge a cell?
2 reasons it doesnt work with non-rechargeable cells
Apply strong electrical force to reverse electron motion + reverse to allow flow of electrons to allow for power output:
Doesn’t work for Non-rechargeable cells:
Forms irreversible products
Reversible reactions producing gaseous products → increasing Pa causing explosions
Give an advantage of rechargeable batteries (other than multiple uses):
Why
Dont have to be thrown away/recycled:
Prevents harmful electrolytes + metals decomposing into the atmosphere
Describe a Lead - Acid Battery
Electrodes
Electrolytes
-ve electrode = Pb
+ve electrode = PbO2 → oxygen is lost to form water molecules
Electrolyte = H2SO4 → Hydrogen ions forms water with oxygen from +ve electrode
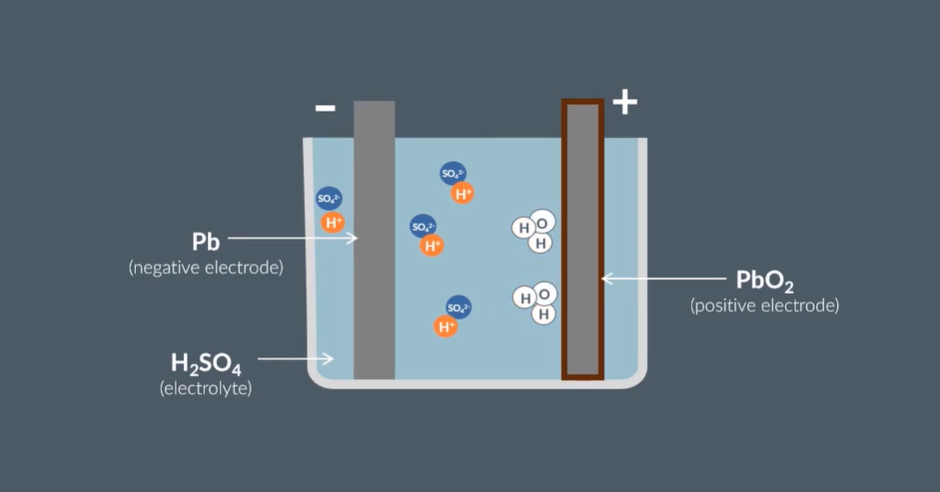
Give the half equation that occurs at the Cathode (+ve)
What type of reaction is it?
What happens when the electrode is “used up”
PbO2(s) + 4H+ + 4e- → Pb(s) + 2H2O(l)
Reduction
Lead reacts to form PbSO4 like the anode does
Give the half equation that occurs at the Anode (-ve)
What type of reaction is it?
Pb(s) + SO4- → PbSO4(s) + 2e-
Oxidation
Give the net overall ionic equation for a Lead-Acid battery
Pb(s) + PbO2(s) + 4H+ + SO42-(aq) → 2PbSO4(s) + 2H2O(l)
What happens to a Lead-Acid battery when used up?
Electrodes are all coated in Lead Sulfate → since H2SO4 forms ions which react with the electrodes
How do electrons flow in Lead-Acid battery?
The anode (-ve) forms positive lead ion (2+), increasing the number of electrons so they flow to the cathode to form lead atoms
Describe the diagram of a Lithium-Ion battery
Electrodes
Electrolytes
Cathode (+ve) = CoO2
Anode (-ve) = Lithium with graphite powder
Electrolyte = LiPF6
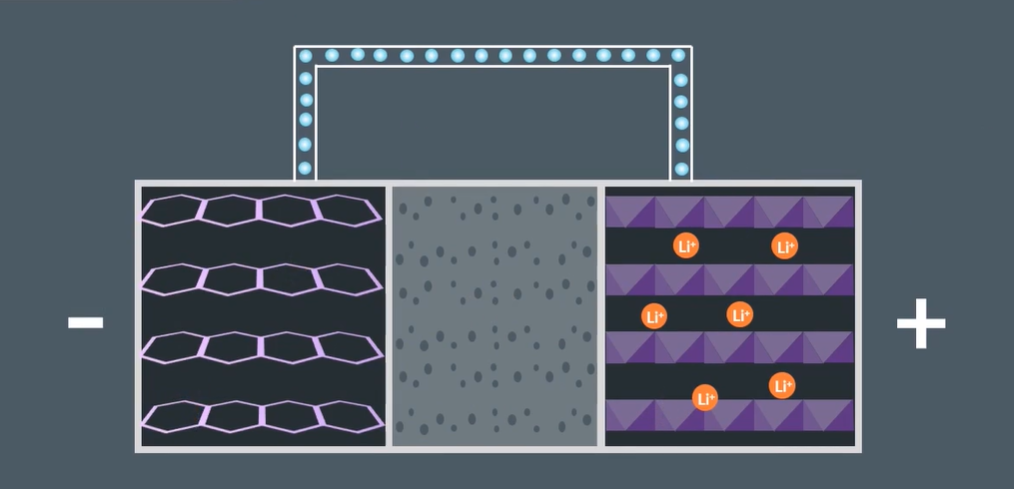
What is the role of Graphite in the Lithium-Ion batteries?
Why?
Graphite powder acts as a support medium:
Allows dissolved Li ions to travel through the electrolyte to the cathode to form LiCoO2
Explain the movement of Li ions to and from electrodes (usage + charging)
Li ions absorbed by graphite allowing them to move easily to the cathode → Li ions inserted in the Cobalt layers to form LiCoO2
When charging they return to the cathode and forms their ions of Li+ and CoO2-, due to the structure of LiCoO2 allows the ions to move through the electrolyte
Give the half equations of a Lithium Ion battery:
Anode
Cathode
Anode (-ve) = Li → Li+ + e-
Cathode (+ve) = CoO2 + e- → CoO2-
What is the state of the electrolyte? (why)
Solid:
Since Li+ and H2O is an explosive reaction in solution/moisture
Give the 4 advantages of Lithium Ion batteries
No water which makes it lighter + prevents rust damage
Per atom Pb produces 2 times the amount of electrons as what is used → makes Li ion battery more efficient
Lithium is a light element
Lithium is a very strong reducing agent → produces higher PD
What is a Fuel Cell?
Battery which is continuously fed reactants so that it supplies PD without being used up over time
Describe the Hydrogen Fuel cell
Reactants
Electrolytes
Hydrogen gas in (Anode -ve)
Oxygen gas in (Cathode +ve)
Water leaves
Electrolyte = KOH/NaOH
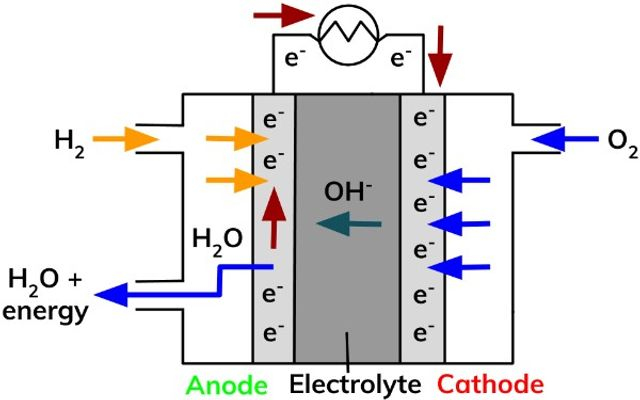
Describe the process that Hydrogen goes through the Hydrogen Fuel cell (Alkaline)
Equation
Which electrode
Hydrogen is oxidised in the anode to form H+ which enter the electrolyte to react with OH to form water and electrons (which go to the cathode):
H2 + 2OH- → 2H2O + 2e-
Anode
Describe the process that Oxygen goes through the Hydrogen Fuel cell (Alkaline)
Equation
Which electrode
Oxygen reduces to Oxide ions (O2-) reacts with H2O to form the OH- ions for the electrolyte:
O2 + 2H2O + 4e- → 4OH-
Cathode
Give the overall equation for an Alkaline Hydrogen fuel cell
2H2 + 2O2 → 2H2O
What are the 2 equations that occur at a Hydrogen fuel cells?
Anode (Oxidation):
H2 → 2H+ + 2e-Cathode (Reduction):
O2 + 4H+ + 4e- → 2H2O
What is the electrolyte in an Acidic Hydrogen Fuel cell?
H3PO4
Give the summary for equations at the Anodes + Cathodes of:
Acidic fuel cell
Alkaline fuel cell
Acidic Fuel Cell:
> Anode (Oxidation) - - - H2 → 2H+ + 2e-
> Cathode (Reduction) - - - O2 + 4H+ + 4e- → 2H2O
Anode makes Ions / Cathode makes waterAlkaline Fuel Cell:
> Anode (Oxidation) - - - H2 + 2OH- → 2H2O + 2e-
> Cathode (Reduction) - - - O2 + 2H2O + 4e- → 4OH-
Anode makes water/ Cathode makes ions
Both Anodes involve Hydrogens, Cathodes involve Oxygen
Give the 3 advantages of Hydrogen fuel cells
Only byproduct is water
Refuelling is quicker than recharging
Recharging involves electrolysis means no carbon emissions
What is the Method to know how to write:
Acid Hydrogen fuel cell Half equations
Alkaline Hydrogen fuel cell Half equations
W ACid = Water, Acidic, Cathode
ALAW it CUW? = Alkaline Anode water - Cathode uses water
Give the 3 disadvantages of Hydrogen fuel cells
Production of cells use machines + electricity which release emissions
Space consuming
H2 is flammable so risky to be in heat
Rank the efficiency of:
Battery
Petrol engines
Hydrogen Fuel cell
Battery > Hydrogen Fuel cell > Petrol engine
How can we increase the efficiency of petrol engine efficiency?
2 advantages
Hydrogen is combusted in petrol engines, increasing efficiency:
Water byproduct
Increased efficiency → better than normal petrol (ONLY)