Thermodynamics
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
What is thermodynamics derived from? And what does it mean?
Thermodynamics is derived from Greek word therme, ‘heat’, and dynamis, ‘power’ or ‘energy’.
It means the flow of heat in physical and chemical reactions
What is thermodynamics? (definition)
Thermodynamics is a branch of science which deals with the study of different forms of energy and their interconversions
What is a system?
A system is a portion of the universe that has been chosen for studying the changes within it in response to varying conditions
Open system
mass and energy can be exchanged with surroundings, e.g., if some water is kept in an open vessel or hot tea in an open cup.
Closed system
In a closed system, there is only the exchange of energy with surroundings, no exchange of mass takes place.
For example, if water is placed in closed metallic vessel r hot tea placed in closed tea pot.
Isolated system
There is neither exchange of mass nor energy with surrounding.
For example, water in a closed and insulated vessel or tea in a thermoflask.
Homogenous system
All the constituents are present in the same phase and the composition of system is uniform throughout the system
Heterogenous system
It contains two or more phases and the composition is uniform throughout
Extensive properties
• Depend on the Quantity of matter present in the system
• Examples: volume, energy, heat capacity, entropy
Intensive properties
• They do not depend on the size of the system or quantity of matter present in it.
• They are dependent on the nature of substance present in it.
• Example: pressure, temperature, density, surface tension
What is process?
When a thermodynamic system changes from one state to another, the operation is called a Process
What do the processes involve?
change of conditions (temperature, pressure and volume)
The first and last state of processes are called-
initial and final state respectively
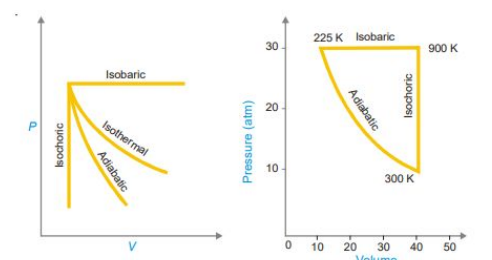
Isothermal process
• It is defined as the process in which temperature of system remains constant.
• Heat can flow from system to surrounding and vice versa in order to keep the temperature constant.
• This is often achieved by placing the system in a thermostat (a constant temperature bath).
• For an isothermal process dT = 0.
Adiabatic process
• Those processes in which no heat can flow into or out of the system, are called adiabatic processes.
• Adiabatic conditions can be approached by carrying the process in an insulated container such as ‘thermos’ bottle.
• High vacuum and highly polished surfaces help to achieve thermal insulation.
• For an adiabatic process dq = 0
Isochoric process
• Those processes in which the volume remains constant are known as isochoric processes.
• The heating of a substance in a non-expanding chamber is an example of isochoric process.
• For isochoric processes dV = 0.
Isobaric process
• Here pressure of the system remains constant during the process
• Those processes which take place at constant pressure are called isobaric processes.
• For example, heating of water to its boiling point and its vaporisation take place at the same atmospheric pressure.
• These changes are, therefore, designated as isobaric processes and are said to take place isobarically.
• For an isobaric process dp = 0
Cyclic process
• When a system in a given state goes through a number of different processes and finally returns to its initial state, the overall process is called a cycle or cyclic process.
• For a cyclic process dE = 0, dH = 0.
Reversible process
• Such a process is carried out infinitesimally slowly so that all changes occurring in the direct process can be reversed and the system and the surrounding remain in state of equilibrium
• It is an ideal process and cannot be realized in actual process
Irreversible process
• Change is brought about rapidly and the system does not attain equilibrium
• The force which drives the reactants towards products is greater than opposing force which is to carry reverse process
Spontaneous process
• It may also be defined as the process which can take place by itself or initiation
Which takes place by itself
• Evaporation of water in open vessel
• Dissolution of salt in water
• Flow of water down a hill
Which takes place by initiation
• Combination of oxygen and hydrogen to form water
• Lighting of candle is initiated by ignition
Non spontaneous process
Does not take place by itself or initiation
• Flow of heat from a cold body to a hot body
• Flow of water up the hill
• Dissolution of sand in water
Internal energy
The total of all possible kinds of energy of a system, is called its internal energy
Internal energy is a state function and classed as an extensive property
Enthalpy
Enthalpy or heat content of a system may be defined as the sum of the internal energy and the product of its pressure and volume
H = E + PV
Where E is the internal energy
P is pressure
V is the volume of the system
Change in enthalpy
It is the difference in the enthalpies of the products and the reactants
∆H = H(products)- H(reactants)
= Hp- Hr
First Law of Thermodynamics
The total energy of an isolated system remains constant though it may change from one form to another
When a system is changed from state A to state B, it undergoes a change in the internal energy from EA to EB. Thus, we can write
deltaE = EB - EA
The change in internal enery of a system is equal to the heat added to the system minus the work done by the system
delta Ee = q- w [q= the amount of heat supplied to the system; w= work done by the system]
![<p>The total energy of an isolated system remains constant though it may change from one form to another</p><p>When a system is changed from state A to state B, it undergoes a change in the internal energy from E<sub>A </sub> to E<sub>B. </sub>Thus, we can write<br>deltaE = E<sub>B </sub>- E<sub>A</sub><br>The change in internal enery of a system is equal to the heat added to the system minus the work done by the system</p><p>delta Ee = q- w [q= the amount of heat supplied to the system; w= work done by the system]</p>](https://knowt-user-attachments.s3.amazonaws.com/179fcb59-3e51-4309-a225-01b01545a9b9.png)
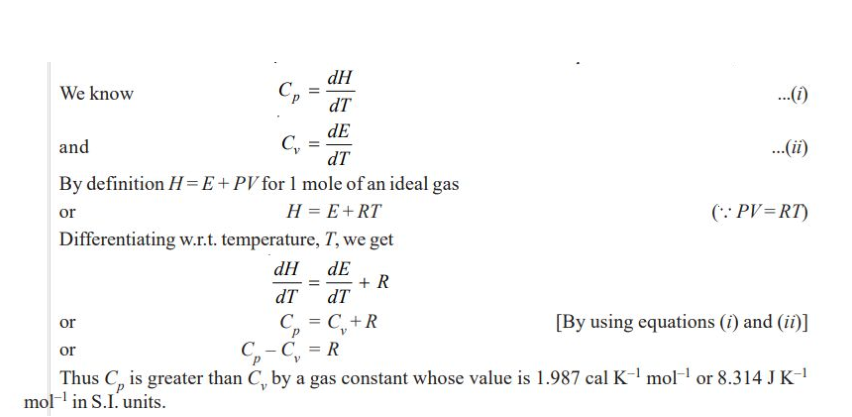
Molar Heat Capacity
Heat capacity of a system is the capacity to absorb heat and store energy.
As the system absorbs heat, it goes into the kinetic motion of the atoms and molecules contained in the system.
This increased kinetic energy raises the temperature of the system
Thus heat capacity of a system is the heat absorbed by unit mass in raising the temperature by one degree (K or ºC) at a specified temperature.
The molar heat capacity of a system is defined as the amount of heat required to raise the temperature of one mole of the substance (system) by 1 K.
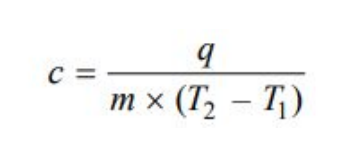
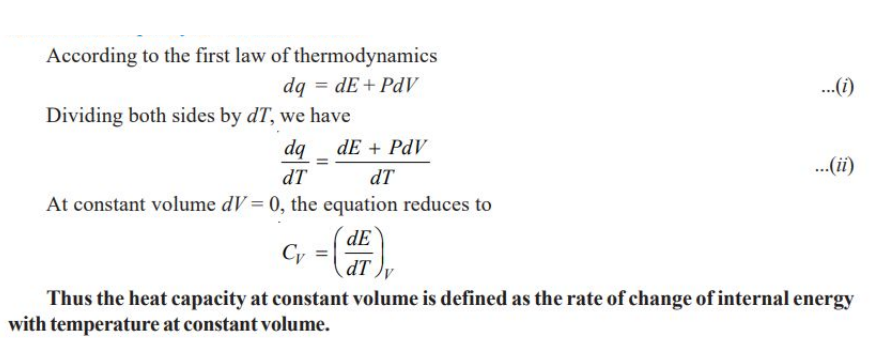
Molar Heat Capacity at Constant Volume
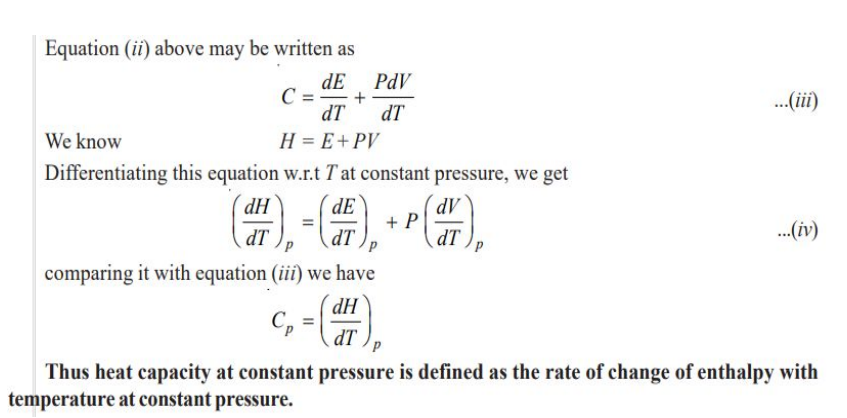
Molar Heat Capacity at Constant Pressure
Relationship between Cp and Cv
Joule-Thomson Effect
The phenomenon of producing lowering of temperature when a gas is made to expand adiabatically from a region of high pressure into a region of low pressure, is known as Joule-Thomson Effect or Joule-Kelvin Effect.
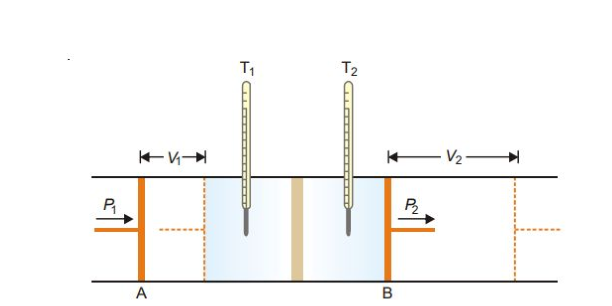
Joule-Thomson Experiment
Explanation. The work done on the gas at the piston A is P1V1 and the work done by the gas at the piston B is P2V2. Hence the net work (w) done by the gas is
w=P2V2 −P1V1
Delta E = q - w (First Law)
But the process is adiabatic and, therefore,q = 0
∴ΔE=E2 −E1 = −w = − (P2V2 −P1V1)
or, E2 − E1 = −(P2V2 −P1V1)
Rearranging,
E2 + P2V2 = E1 + P1V1
H2 = H1 or ΔH=0
Thus the process in Joule-Thomson experiment takes place at constant enthalpy.
Explanation of Joule-Thomson Effect
Joule-Thomson expansion of a gas is carried at constant enthalpy. But
H = E + PV
Since H remains constant, any increase in PV during the process must be compensated by decrease of E, the internal energy. This leads to a fall in temperature i.e., T2<T1. For hydrogen and helium PV decreases with lowering of pressure, resulting in increase of E and T2 > T1. Below the inversion temperature, PV increases with lowering of pressure and cooling is produced
Spontaneous process
A process which proceeds of its own accord, without any outside assistance, is termed a spontaneous or natural process.
Spontaneiity
The tendency of a process to occur naturally is called the spontaneity
Criteria of spontaneity
(1) A spontaneous change is one-way or unidirectional.
(2) For a spontaneous change to occur, time is no factor.
(3) If the system is not in equilibrium state (unstable), a spontaneous change is inevitable.
(4) Once a system is in equilibrium state, it does not undergo any further spontaneous change in state if left undisturbed.
(5) A spontaneous change is accompanied by decrease of internal energy or enthalpy (ΔH).
Entropy
• Entropy is a thermodynamic state quantity that is a measure of the randomness or disorder of the molecules of the system.
• The greater the randomness, the greater the entropy.
• Entropy of a crystalline substance is minimum in the solid state and maximum in the gaseous state.
• Melting of ice is an example of increasing entropy
ΔS = Sfinal - Sinitial
When Sfinal > Sinitial , ΔS is positive
A process accompanied by an increase in entropy tends to be spontaneous
Second Law of Thermodynamics
The second law of thermodynamics states that- Whenever a spontaneous process takes place, it is accompanied by an increase in the total energy of the universe.

3rd Law of Thermodynamics
• At absolute zero (T= 0 K) a perfectly crystalline solid has a perfect order of its constituent particles, i.e., there is no disorder at all.
Hence, absolute entropy is taken as zero (S = 0)
Zeroth Law of Thermodynamics
• When two bodies A and B are separately in thermal equilibrium with a third body, they in turn are in equilibrium with each other
If A ⇌C and B ⇌ C
Then A ⇌B
Importance of thermodynamics
• Useful to predict whether any chemical reaction can occur under specified conditions
• Used to predict the extent of chemical reaction before equilibrium is reached
• Used to derive important laws like Law of equilibrium.
Exothermic reaction
If heat is liberated in a chemical reaction the process is said to be exothermic reaction.
C(s)+O2(g)=CO2 + 94.03kcal
Endothermic reaction
If heat is absorbed in a chemical reaction the process is said to be endothermic reaction.
H2(g)+I2(s) + 11.90Kcal =2HI(g)
Reaction of thionyl chloride (SOCl2) with cobalt (II) sulfate heptahydrate
Endothermic
Making ice cubes
Exothermic
Reaction of barium hydroxide octahydrate crystals with dry ammonium chloride
Endothermic
Formation of snow in clouds
Exothermic
Melting solid salts
Endothermic
Condensation of rain from water vapour
Exothermic
Making an anhydrous salt from a hydrate
Endothermic
Mixing sodium sulfite and bleache
Exothermic
Mixing water and ammonium nitrate
Endothermic
Rusting iron
Exothermic
Seperating ion pairs
Endothermic
Burning sugar
Exothermic
Producing sugar by photosynthesis
Endothermic
Forming ion pairs
Exothermic
Cooking an egg
Endothermic
Mixing water and strong acids
Exothermic
Baking bread
Endothermic
Mixing water with an anhydrous salt
Exothermic
Evaporation of water
Endothermic
Crystallizing liquid salts (as in sodium acetate in chemical handwarmers)
Exothermic
Conversion of frost to water vapour
Endothermic
Nuclear fission
Exothermic
Melting ice cubes
Endothermic
Mixing water with calcium chloride
Exothermic process