Grade 11 Pre-AP Chemistry - Atomic History, Experiments
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
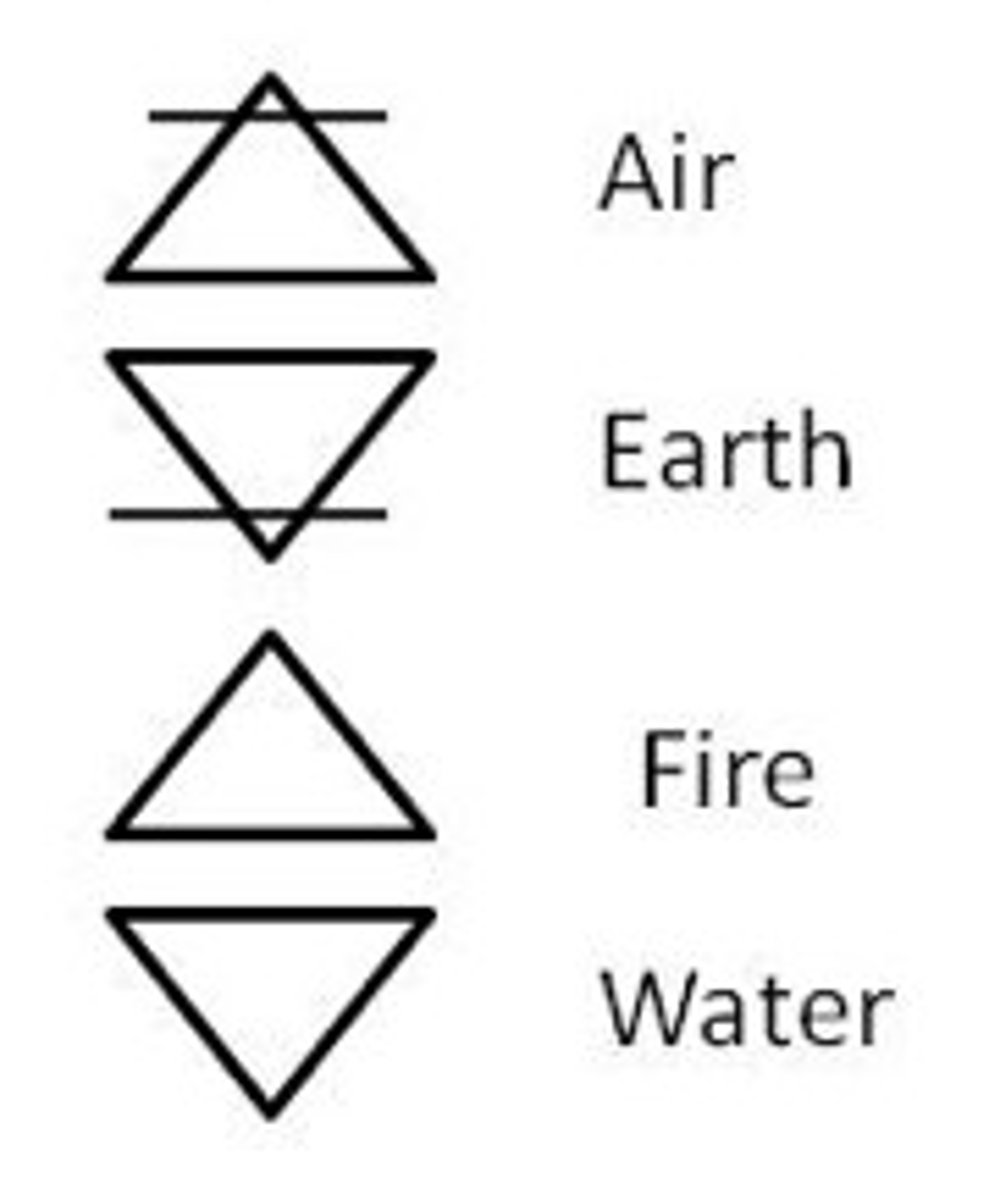
Aristotle's Ideology
only 4 elements: earth, water, wind, fire
believed that matter was continuous and not made up of smaller particles
Democritus' Ideology
believed if you kept cutting a substance in half you would reach a particle that couldn't be cut further
origin of modern idea of atom

Democritus' Errors
-believed all substances had their own particles (e.g. tree particle)
-didn't recognize similarities between different particles

Aristotle vs Democritus
Aristotle's ideology was more believed and popular, even though Democritus' was more correct

John Dalton
-elements are made of indivisible atoms
-atoms of the same element are identical
-atoms can be neither created nor destroyed
(atomic theory)

Dalton's atomic model
plain timbit
J.J. Thomson
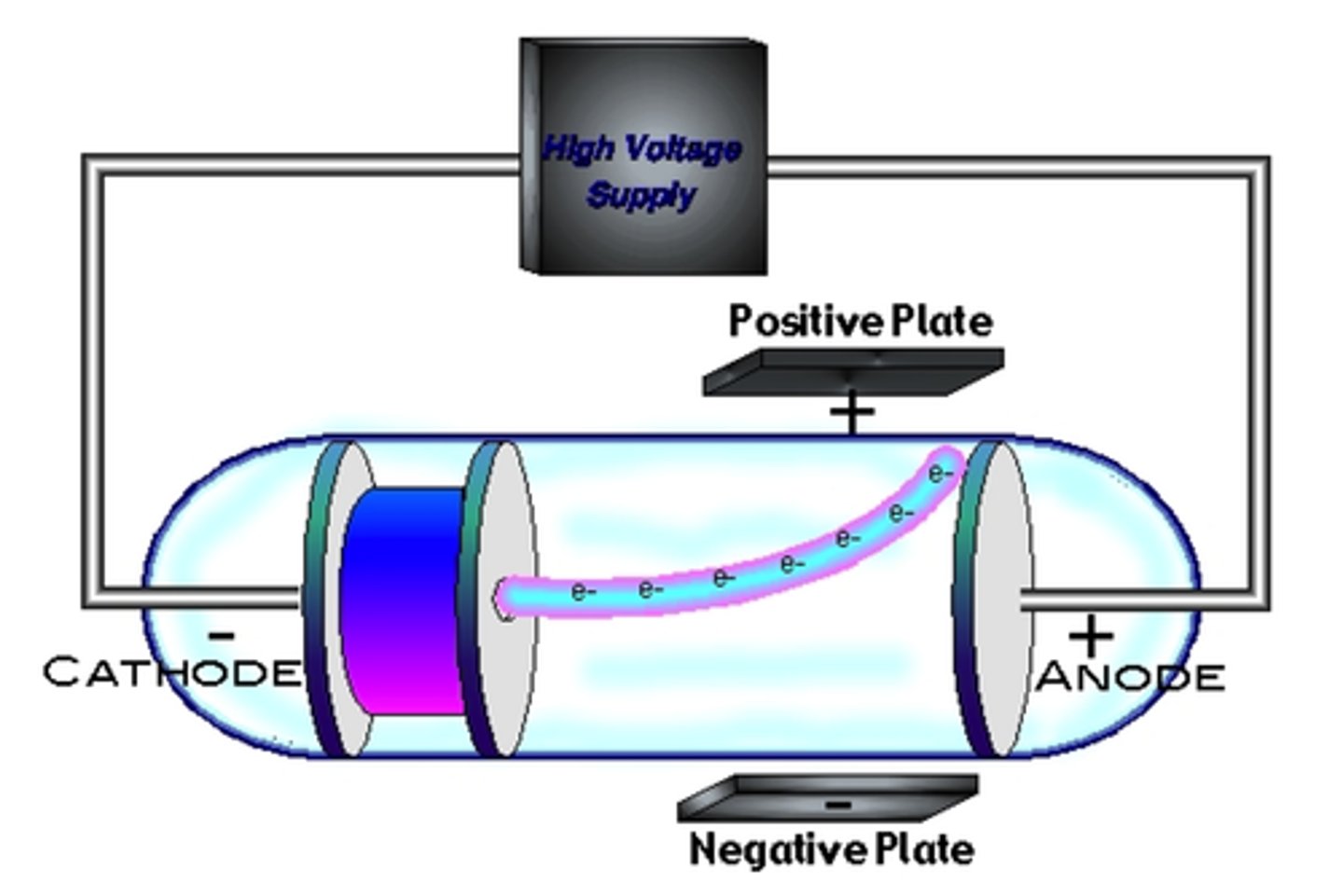
cathode ray tube experiment
discovered electron

cathode ray tube experiment
(+) and (-) plates were placed above and below the CRT
the ray deflected away from the (-) plate, hinting that the particles were (-) charged
when magnetic plates were placed above and below the CRT the ray was again deflected; the particles have mass
applications of the cathode ray tube experiment
old TVs
computer monitors
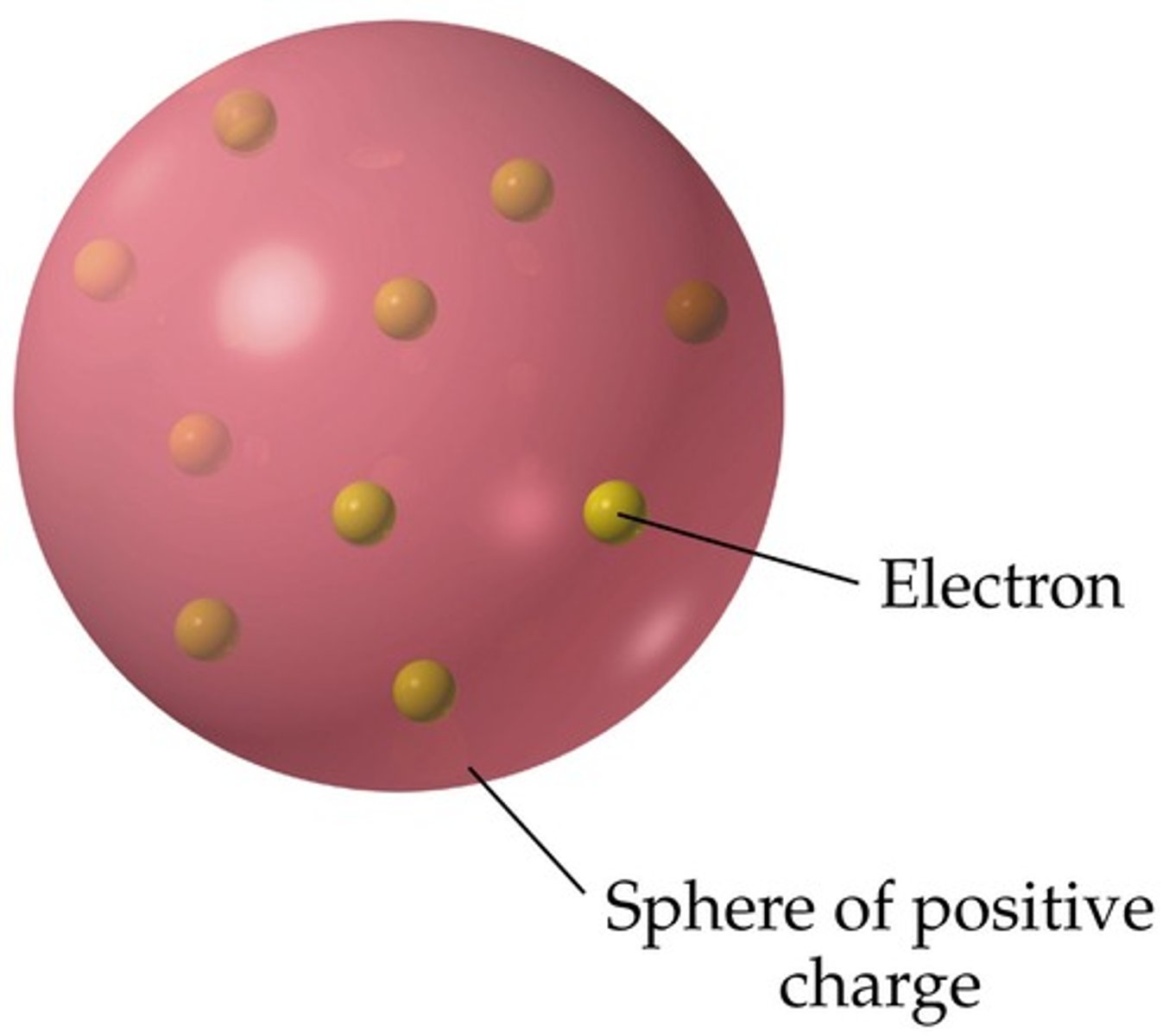
J.J. Thomson atomic model
plum pudding model
electrons just scattered throughout and rest of atom was positive
Ernest Rutherford
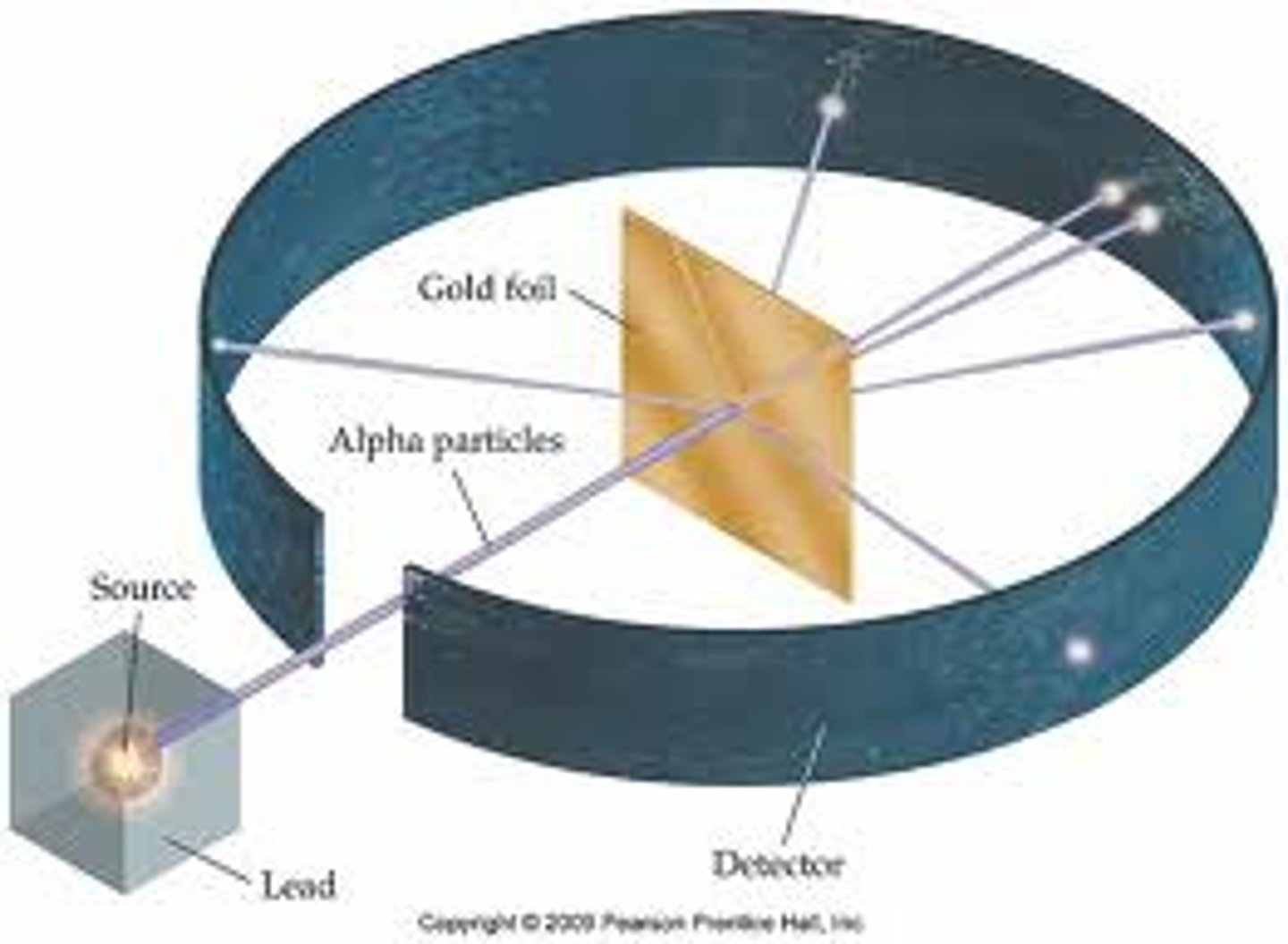
gold foil experiment
discovered nucleus

gold foil experiment
positive particle was shot at gold foil
rays were expected to pass through
most did but some deflected away and some even reflected back towards the origin
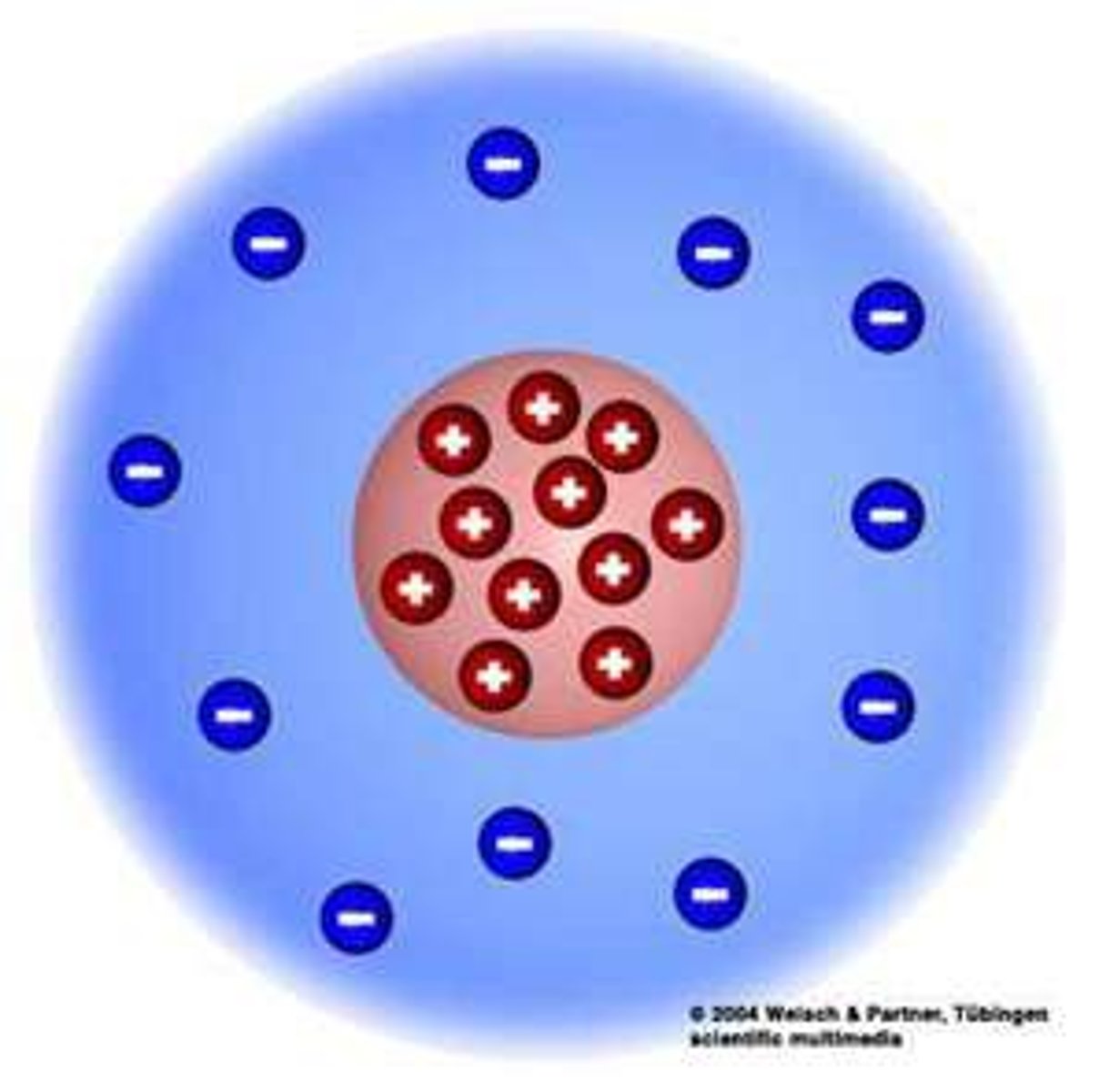
Rutherford atomic model
gold foil experiment discoveries
-most of the atom is empty space (most positive particles went through)
-mass/positive charge concentrated in dense core called nucleus (which is why the positive particle deflected)
-electrons orbit the nucleus like planets orbit the sun
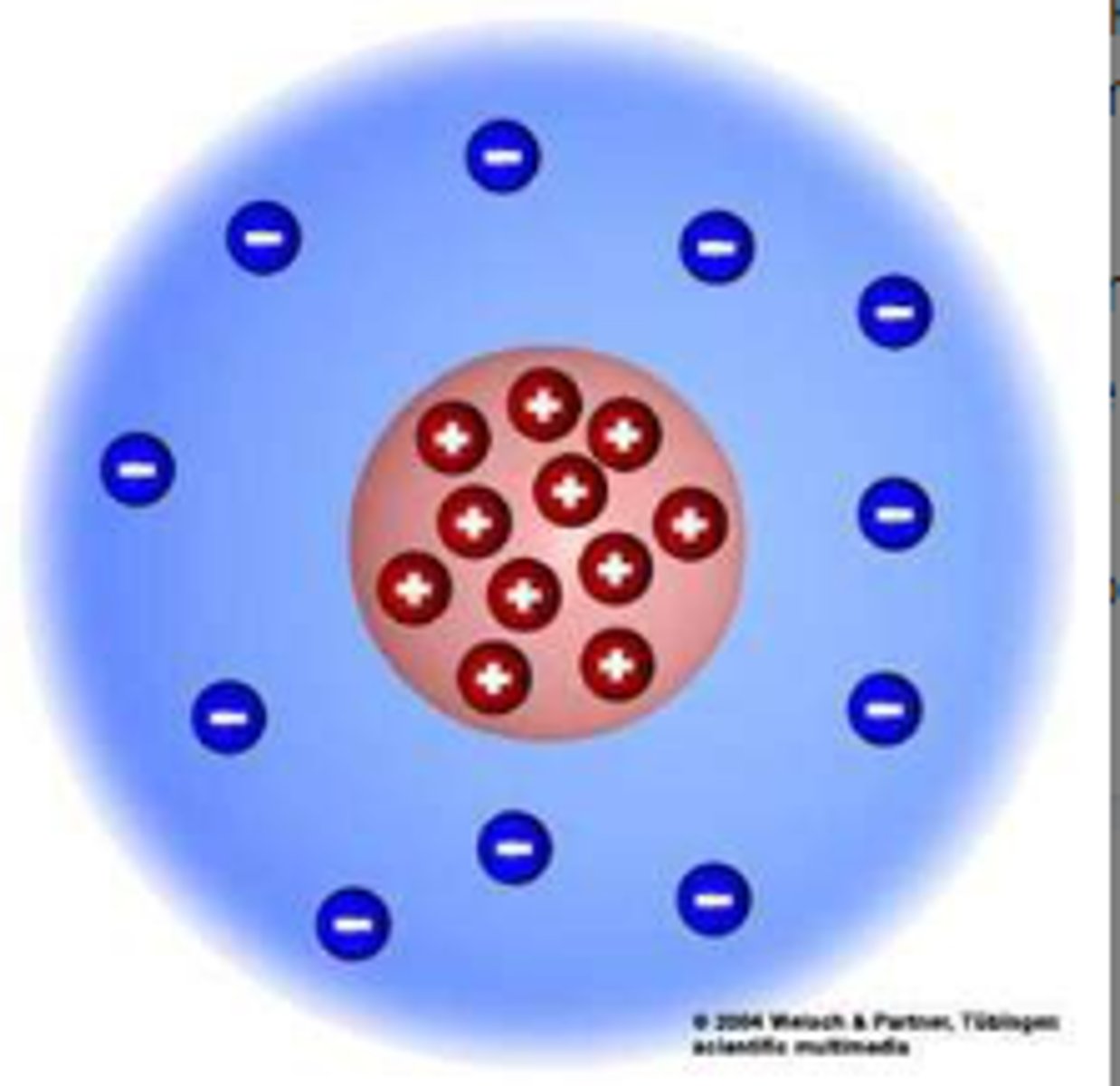
problem with electron orbiting the nucleus
-an object that moves in a circular orbit is constantly accelerating
-an accelerating object releases energy
-object will eventually slow down
-electron will crash into the nucleus
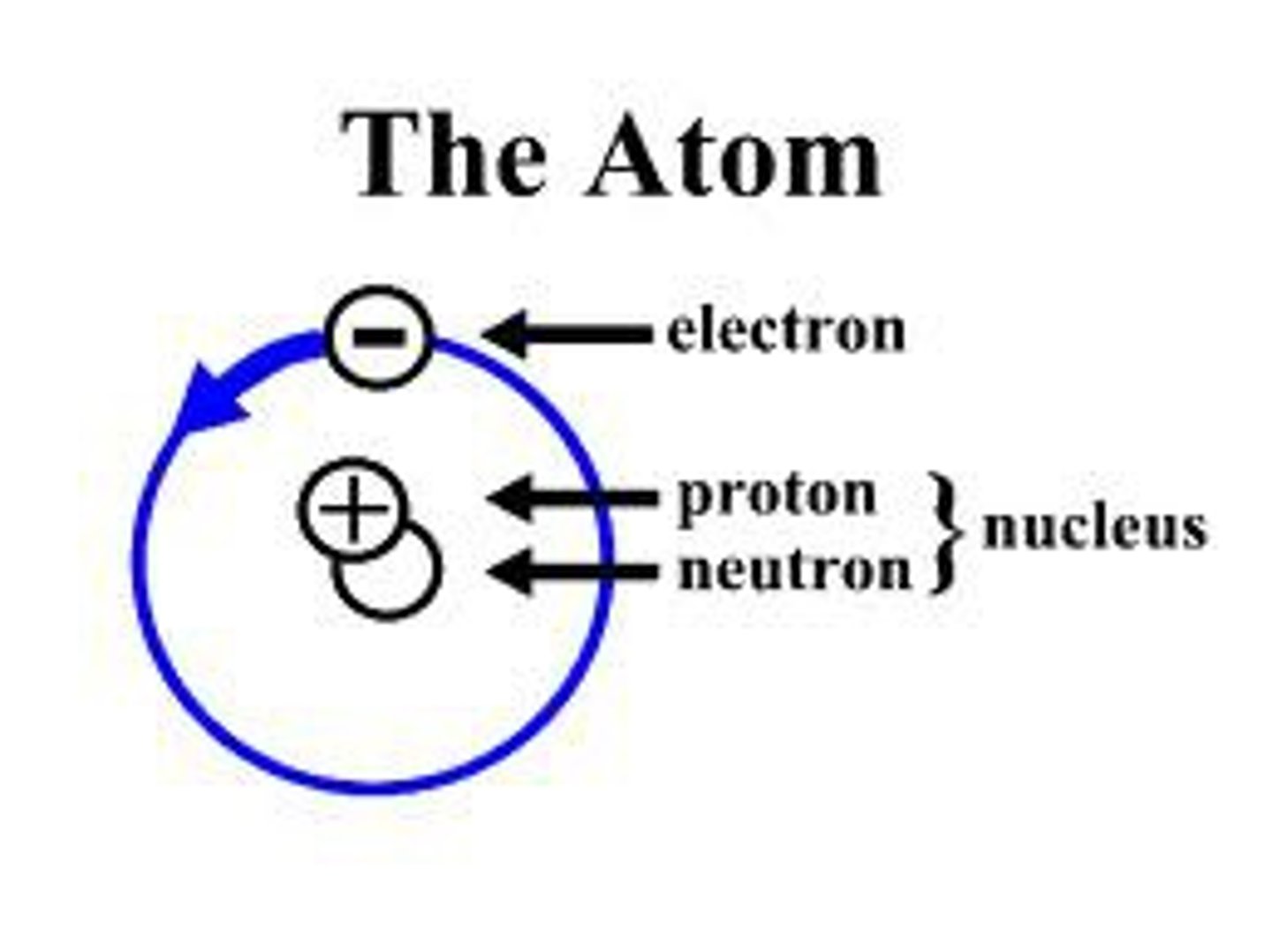
Eugene Goldstein
canal ray tube experiment (same as CRT experiment)
discovered proton

James Chadwick
discovered the neutron

Neils Bohr
discovered that electrons are in energy levels
spectroscopy experiment

spectroscopy experiment
-Bohr studied hydrogen gas
-looked at light produced when H was burned/attached to electricity
-split the light with spectroscope
-saw a set of 4 colored lines
-called it the emission spectrum of H
-speculated that electrons can only exist at certain distances (stationary states/E levels)
-electrons absorb energy when supplied with electricity/heat and move to a higher orbit (excited state)
-electron not stable in excited state so drops back down (ground state)
-relaxes by emitting energy in the form of light (4 lines observed)
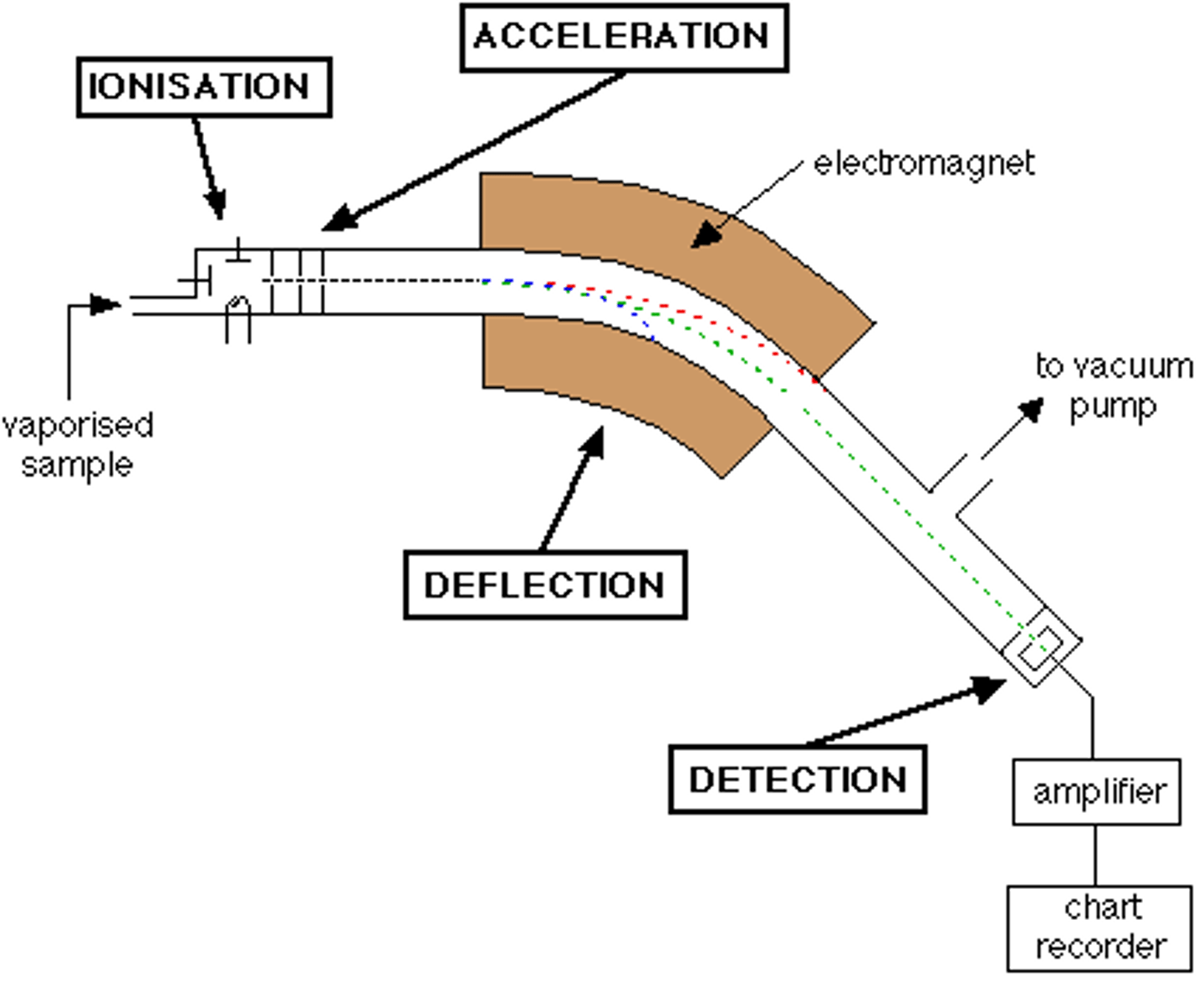
mass spectrometer
particles are separated based on mass by a magnetic field
sample gets heated to turn into gas
hit by an electron beam, knocking off some electrons (+)
1st electric plate is (+), 2nd is (-), pulling the sample forward and accelerate
sample hits magnetic field where they deflect based on their mass
lightest particles deflect most
detection plate coated in phosphorescent material (lights up when particles hit it)
Timeline of Atomic History
Dalton -> Thomson -> Rutherford -> Bohr -> Chadwick